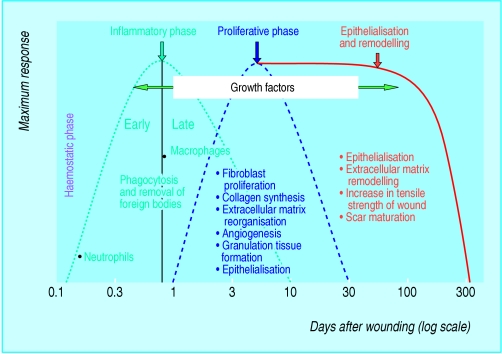
The healing of acute wounds involves a complex and dynamic series of events leading to the repair of injured tissues. These events, triggered by tissue injury, involve four overlapping but well defined phases: haemostasis, inflammation, proliferation, and remodelling.
Figure 1.
The four phases of acute wound healing
Table 1.
Composition of some tissue engineered skin substitutes
| Product by type | Content and description |
|---|---|
| Epidermal | |
| Epicel, Laserskin | Cultured epidermal autograft (sheet) |
| CellSpray | Cultured epidermis in suspension form |
| BioSeed-S | Keratinocyte-fibrin-glue suspension |
| LyphoDerm | Lysate of cultured human keratinocytes, comprising cytokines and growth factors |
| Dermal (acellular) | |
| Integra | Two layered skin substitute comprising biodegradable matrix and bovine collagen, and outer silicone layer |
| AlloDerm | Processed human cadaver skin with acellular dermal matrix and intact basement membrane |
| Biobrane | Porcine dermal collagen bonded to semipermeable silicone membrane |
| Dermal (cellular) | |
| TransCyte | Allogenic human fibroblasts cultured on nylon mesh coated with porcine collagen |
| Dermagraft | Allogenic human fibroblasts cultured on bioabsorbable scaffold |
| Composite | |
| Apligraf, OrCel | Allogenic cultured skin containing keratinocytes, fibroblasts, and bovine collagen |
Haemostasis is secured by platelet aggregation and clot formation. The inflammatory phase begins with the arrival of phagocytic neutrophils and, later, macrophages at the wound site; they are important sources of and substrates for growth factors. The proliferative phase is characterised by the formation of new blood vessels (angiogenesis), synthesis of extracellular matrix components such as collagen, granulation tissue formation, and re-epithelialisation. The extracellular matrix is continually remodelled during the final phase; an avascular scar is the end result of the healing process.
Table 2.
Tissue engineered skin substitutes in wound healing
| Product | Uses/advantages | Disadvantages |
|---|---|---|
| Epicel, Laserskin * | Permanent coverage for superficial and partial thickness burns | 2-3 week lag period between biopsy and obtaining epidermis; lacks dermal component |
| Integra† | Immediate permanent coverage for surgically excised full thickness burns; reconstructive surgery | Requires healthy and non-infected wound base; in burns, autograft is needed after 3-4 weeks for epithelial cover |
| AlloDerm† | Intended to permanently cover full thickness burns and deep ulcers; reconstructive surgery | In burns, may necessitate removal after 2-3 weeks; autograft is needed for epithelial cover; not suitable for infected wounds |
| Biobrane† | To cover extensive partial thickness burns and donor sites | Temporary; not suitable for infected burn wounds |
| TransCyte‡ | To cover surgically excised full thickness burns and non-excised partial thickness burns | Temporary (may need skin grafting after 2-3 weeks); not suitable for infected wounds and patients allergic to porcine collagen |
| Dermagraft‡ | Non-healing diabetic foot ulcer and venous leg ulcer | Not for infected wounds or ulcers with sinus tracts |
| Apligraf§ | Non-healing diabetic foot ulcer and venous leg ulcer | Not for infected wounds or patients allergic to bovine collagen |
| OrCel§ | Acute and chronic deep dermal ulcers, partial thickness burns and donor site wounds | Not for infected wounds or patients allergic to bovine collagen |
*Epidermal; †Dermal, acellular; ‡Dermal, cellular; §Composite.
Chronic wounds may be arrested in any of the four phases; commonly, however, disruption occurs in the inflammatory or the proliferative phases. Many mediators—including inflammatory cells, growth factors, proteases such as matrix metalloproteinases (MMPs), and cellular and extracellular elements—play important roles in different stages of the healing process. Alterations in one or more of these components may account for the impaired healing observed in chronic wounds.
Biological based treatments
Cryopreserved human cadaver skin (used in the UK), and human amniotic membrane and frog skin (used in other parts of the world) have long been used to treat wounds, particularly burns. More recently, artificial “skin substitutes” and growth factors have been developed to help achieve healing in chronic, non-healing wounds of varying aetiologies. These treatments target different stages of the healing process and, in the case of skin substitutes, replace lost tissue.
Artificial skin substitutes, products of tissue engineering, consist of a microengineered, biocompatible, polymer matrix in combination with cellular and/or extracellular elements such as collagen. Several growth factors (proteins involved in coordinating and regulating various interrelated processes during wound healing) produced by recombinant DNA technology have also been developed to aid healing of such wounds.
This is the last in a series of 12 articles
Products targeting inflammatory phase
The production and activity of several proteases—including metalloproteinases, serine proteases, and neutrophil elastases, which are tightly regulated in acute wound healing—may be altered in chronic wounds. Raised levels of such proteases can be detrimental to wound healing, and products aimed at counteracting their effect have been developed. One such product is Promogran, which is designed to inactivate proteases and also protect the host's naturally produced growth factors. It may be useful in the treatment of chronic wounds refractory to conventional treatments, but it is not effective in infected wounds or those with unhealthy wound beds.
Figure 2.
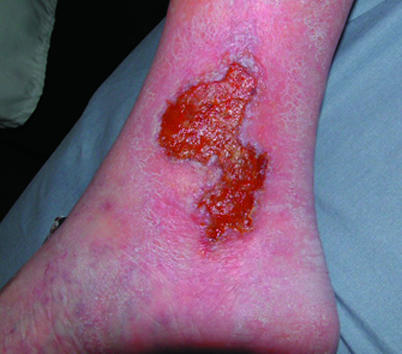
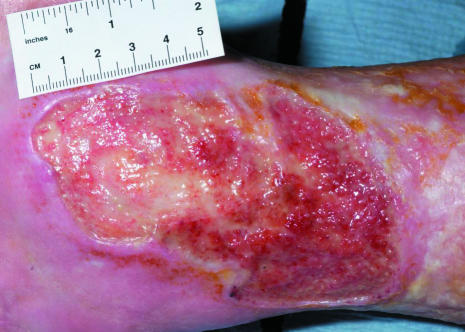
Left: Chronic venous leg ulcer suitable for protease inhibitor dressing. Right: Infected diabetic foot ulcer, associated with Charcot's arthropathy, suitable for G-CSF treatment
Table 3.
Selected growth factors in wound healing*
| Growth factor | Wound healing related function | Indication |
|---|---|---|
| VEGF | Stimulates angiogenesis and collateral blood vessel development; accelerates granulation tissue formation | Has potential for use in diabetic ulcers; gene transfer encoding VEGF shown to be effective in ischaemic legs and ulcers by formation of collateral blood vessels |
| FGF | Promotes fibroblast proliferation, matrix deposition, wound contraction, and angiogenesis | Trials show that topical recombinant bovine FGF is effective in burns, donor site wounds, and pressure ulcers |
| KGF | Promotes proliferation and migration of keratinocytes | Trials show that topical recombinant human KGF-2 (repifermin) is effective in venous leg ulcers |
| EGF | Stimulates keratinocyte differentiation, proliferation, migration, and adhesion | Trials show that topical recombinant human EGF is effective in partial thickness burns |
| PDGF | Chemoattractant for neutrophils and fibroblasts; stimulates fibroblast proliferation | Topical recombinant human PDGF-BB (becaplermin) shown to be effective in diabetic foot ulcers |
| G-CSF | Stimulates production of neutrophils; enhances neutrophil and monocyte function; promotes keratinocyte proliferation | Recombinant human G-CSF injected subcutaneously shown to be effective in infected diabetic foot ulcers |
| GM-CSF | Mediates epidermal cell proliferation | Trials show that topical recombinant human GM-CSF enhances healing of venous leg ulcers |
| HGF | Recruits neutrophils, monocytes, and mast cells; has mitogenic and morphogenetic properties | Topical recombinant HGF may accelerate healing of chronic venous leg ulcers |
| TGF-β | Attracts macrophages and fibroblasts to wound site; stimulates angiogenesis and collagen metabolism | Trails show that recombinant human TGF-β2 is effective in diabetic foot ulcers |
VEGF = vascular endothelial growth factor; FGF = fibroblast growth factor; KGF = keratinocyte growth factor; EGF = epidermal growth factor; PDGF = platelet derived growth factor; HGF = hepatocyte growth factor; G-CSF = granulocyte colony stimulating factor; GM-CSF = granulocyte macrophage colony stimulating factor; TGF-β = transforming growth factor β. *Only PDGF is licensed for commercial use in the United Kingdom.
Products targeting proliferative phase
Growth factors
Fibroblasts, the key type of cell in the healing process, are attracted to the wound site by several growth factors, including platelet derived growth factor (PDGF) and TGF-β. They proliferate and produce the matrix proteins fibronectin, hyaluronan, and later, collagen and proteoglycans, all of which help to construct the new extracellular matrix.
Figure 3.
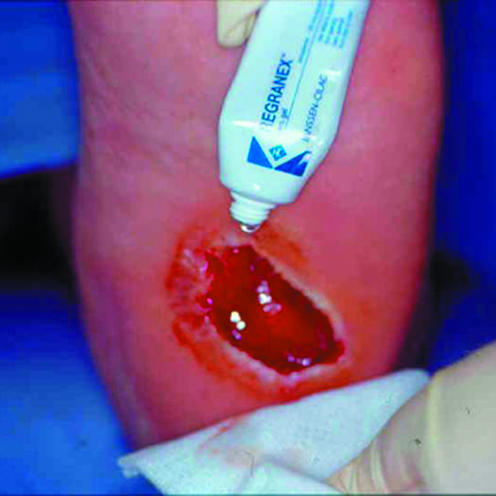
Topical application of recombinant platelet derived growth factor on a diabetic foot ulcer
Growth factors, including granulocyte colony stimulating factor (G-CSF) and transforming growth factor-β (TGF-β), have also been used to target this phase of healing. G-CSF, an endogenous haemopoietic growth factor, induces terminal differentiation and release of neutrophils from the bone marrow, enhances neutrophil and macrophage function, and promotes keratinocyte proliferation. Recombinant human G-CSF, injected subcutaneously, has been shown to enhance healing in infected diabetic foot ulcers.
TGF-β is chemotactic for macrophages, induces the production of collagen and fibronectin, and inhibits metalloproteinase activity. TGF-β1 has been shown to accelerate wound healing in animal models, and topical application of TGF-β2 has been shown to be effective in the healing of diabetic foot ulcers
PDGF attracts keratinocytes and promotes the formation of granulation tissue. Recombinant PDGF was developed to expedite the proliferative phase. Becaplermin (as Regranex gel) is the only growth factor currently licensed for commercial use in the United Kingdom. A multicentre, double blind randomised controlled trial in patients with chronic diabetic foot ulcer showed topical PDGF to be superior to placebo in promoting healing. Its effectiveness was further enhanced when used in conjunction with debridement of the wound bed, emphasising the importance of good basic wound care.
Figure 4.
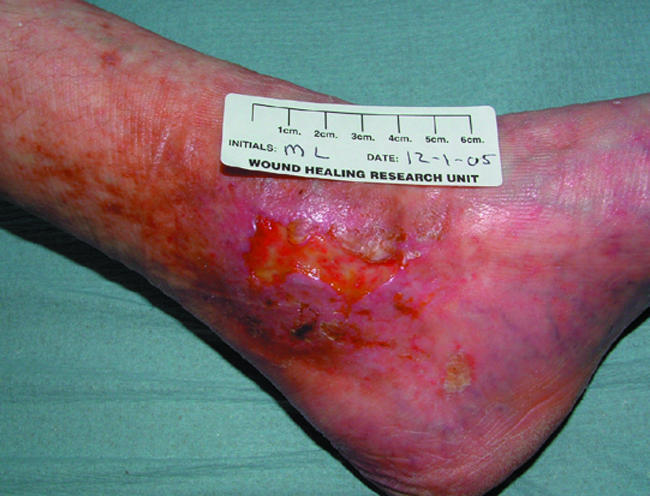
Venous leg ulcer suitable for use of dermal or composite skin substitute
Fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) are active in this phase of repair. FGF promotes fibroblast proliferation and collagen accumulation and accelerates the formation of granulation tissue. VEGF plays a crucial role in angiogenesis.
Figure 5.
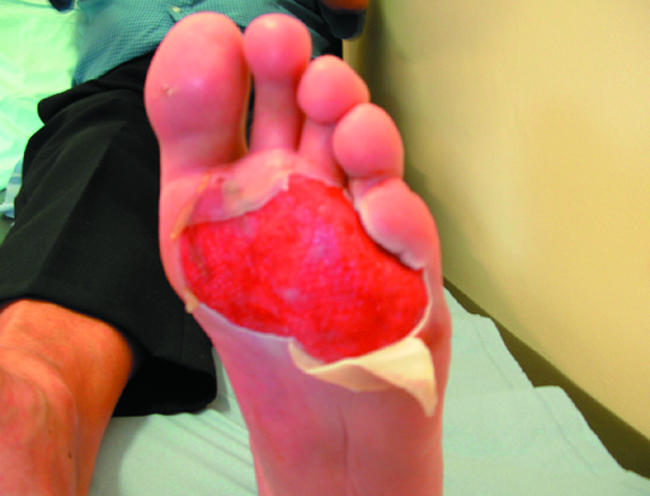
Partial thickness burn suitable for an epidermal skin substitute
Cell and matrix based treatments
Autologous fibroblasts (that is, from the patient's own dermis) seeded onto a matrix derived from hyaluronic acid have been shown to be useful in treating diabetic foot ulcers and venous leg ulcers. Similarly, acellular collagen based matrices designed to mimic the extracellular matrix have been successfully used to treat chronic ulcers of varying aetiologies.
Figure 6.
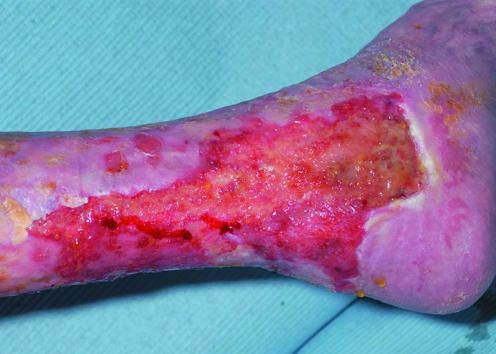
Wounds suitable for therapies aimed at the remodelling and epithelialisation phases of wound healing
Loss of the dermal layer occurs frequently in deep ulcers and burns. Allogenic fibroblasts, obtained from neonatal human foreskin and cultured in vitro, have been used to provide the dermal replacement in such wounds. They are seeded either on a biologically absorbable scaffold (for example, Dermagraft) or on a nylon mesh (for example, TransCyte). The proliferating fibroblasts secrete collagen, matrix proteins, and growth factors and promote healing. They are designed to provide dermal replacement in a variety of wounds, though most evidence to date comes from the treatment of diabetic foot ulcers and burns.
Figure 7.
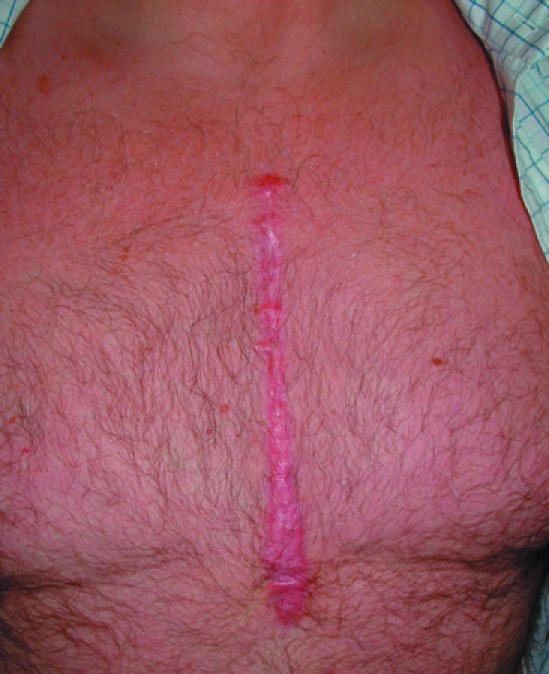
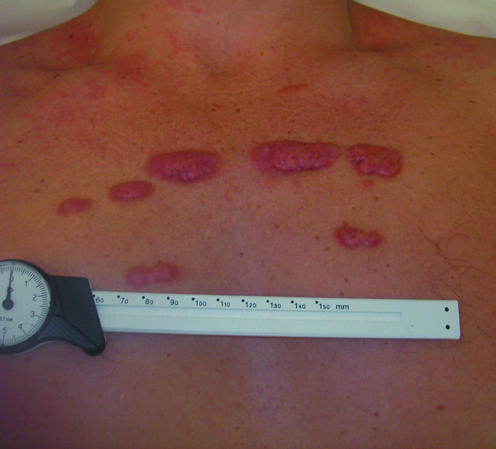
Left: Hypertrophic scar complicating healing of a sternotomy wound. Right: Keloid scarring of acne in an adolescent
Processed human cadaver skin, in which the cells are removed to leave a non-antigenic dermal scaffold (for example, AlloDerm), was one of the first dermal replacement treatments. Composite skin substitutes comprising allogenic keratinocytes (epidermal equivalent) and fibroblasts (dermal equivalent) are also effective in treating diabetic foot ulcers and venous leg ulcers.
Products targeting epithelialisation and remodelling
Growth factors
Epidermal growth factor (EGF) plays a vital role in keratinocyte differentiation, proliferation, migration, and adhesion. Topical application of recombinant EGF is effective in inducing epithelialisation of partial thickness burns and superficial granulating wounds. Keratinocyte growth factor (KGF) also induces proliferation and migration of keratinocytes. Recombinant human KGF-2 induces proliferation of epithelial cells and has been shown to enhance healing of venous leg ulcers. Granulocyte macrophage colony stimulating factor (GM-CSF), secreted by keratinocytes shortly after injury, mediates epidermal cell proliferation in an autocrine manner. Topical recombinant human GM-CSF is effective in the healing of venous leg ulcers.
TGF-β3 causes reduced deposition of collagen during the proliferative and remodelling phases, thus reducing scar formation. Trials into the efficacy of TGF-β3 in the treatment of hypertrophic scars are under way; the role of TGF-β3inthe treatment of keloid scars is unclear.
Cell based treatments
Autologous keratinocyte grafts or suspension (obtained after biopsy and culture of the individual's own keratinocytes) and allogenic cultured keratinocyte grafts are used to treat diabetic foot ulcers, venous leg ulcers, and partial thickness burns. A keratinocyte suspension in a fibrin sealant matrix has recently been developed to aid adherence of the keratinocytes to the wound bed (keratinocyte-fibrin-glue suspension). In addition, a total lysate of cultured human keratinocytes—comprising growth factors, cytokines, and matrix molecules, in a hydrophilic gel—has been developed to treat non-healing venous leg ulcers. The keratinocyte products are primarily used in specialist centres in the treatment of chronic wounds and burns.
Recombinant bovine FGF accelerates the formation of granulation tissue and epidermal regeneration in patients with pressure ulcers and burns. Topical VEGF improves angiogenesis and granulation tissue formation in ischaemic wounds in animal models. Intramuscular gene transfer of VEGF has been shown to improve the collateral circulation in patients with peripheral vascular disease, leading to healing of ischaemic ulcers
Delivery of growth factors
Growth factors are currently delivered to the wound either topically (such as platelet derived growth factor) or by subcutaneous injections (such as granulocyte colony stimulating factor). However, their effectiveness is limited owing to their short half life and the presence of proteases in chronic wounds. To overcome this, gene therapy (the transfer of nucleic acid) has been investigated as a means of providing a longer lasting source
Concerns about biological products
Lack of level 1 evidence
Randomised controlled trials are lacking for many biological products, and the current evidence for many biological based treatments is based on non-randomised prospective trials, retrospective reviews, small case series (institutional or personal), and isolated case reports. Furthermore in the case of growth factors, there is little evidence on dose and duration of treatment.
Table 4.
Products targeting different phases of wound healing process
| Phase | Product | Growth factors |
|---|---|---|
| Inflammatory phase | Promogran | G-CSF, TGF-β1 and β2 |
| Proliferative phase | Dermagraft, TransCyte, Alloderm, Apligraf, OrCel | PDGF, FGF, VEGF |
| Epithelialisation | Epicel, Laserskin, CellSpray, BioSeed-S, LyphoDerm, Trancell | EGF, KGF, GM-CSF |
| Remodelling and scarring | Silicone based products, such as silicone gel sheet (Cica-care) | TGF-β3 |
For abbreviations, see footnote to previous table.
No trials have evaluated the effectiveness of two comparable products (for example, dermal versus dermal skin substitute). Large, multicentre double blind randomised trials are ongoing for some of the products.
Table 5.
Basics of advanced wound care
| • Tissue engineered skin substitutes and other biological wound manipulations are seldom effective in sloughy and exudative wounds with unhealthy wound beds |
| • Good wound care (wound debridement and exudate management), adequate rest, compression, pressure relief, and skin care must be provided as clinically indicated |
| • The new approaches and biological based treatments should complement, not replace, the tenets of good, basic wound care |
Transmission of diseases
Before human tissue is obtained for use in tissue engineered skin substitutes, the donors' medical histories are extensively reviewed and blood samples are screened for a wide variety of infectious diseases, including hepatitis, HIV, and syphilis. However, products obtained from human sources cannot be terminally sterilised owing to the presence of viable human cells, and existing tests cannot provide absolute assurance that such products will not transmit unknown diseases.
Similarly, tissue (for example, serum, collagen, cells) obtained from animal sources also carries the theoretical risk of transmitting infection, particularly prion diseases such as Creutzfeldt-Jakob disease.
Other concerns
Most biological based products contain bovine, porcine, or human constituents and thus have religious and ethical implications.
Tissue engineered skin substitutes and growth factors produced by recombinant DNA technology are expensive, which may limit their widespread use.
Current areas of research include regulation of target genes of cells involved in wound healing; new methods of delivery of specific cell products to the wound (including nanotechnology); use of adult pluripotent stem cells, which are capable of differentiating into essential cells involved in wound healing (such as fibroblasts, endothelial cells, keratinocytes). To date, there is only experimental (though promising) evidence for gene and stem cell therapy in the treatment of chronic wounds
Cutaneous wound healing is a multistep process requiring the interaction and coordination of many different cell types and molecules—including growth factors and proteases. Given the multiple molecular mechanisms involved, no single mediator, growth factor, or gene is likely to be successful in accelerating healing. Similarly, there is heterogeneity within wound types; identification of the cellular and molecular dysfunction in individual wounds and targeting or supplementing them is one of the goals for the future
The ABC of wound healing is edited by Joseph E Grey (joseph.grey@cardiffandvale.wales.nhs.uk), consultant physician, University Hospital of Wales, Cardiff and Vale NHS Trust, Cardiff, and honorary consultant in wound healing at the Wound Healing Research Unit, Cardiff University, and by Keith G Harding, director of the Wound Healing Research Unit, Cardiff University, and professor of rehabilitation medicine (wound healing) at Cardiff and Vale NHS Trust. The series will be published as a book in summer 2006.
Competing interests: For series editors' competing interests, see the first article in this series.
The wound healing chart is adapted from Clark RA. In: Goldsmith LA, ed. Physiology, biochemistry and molecular biology of the skin. 2nd ed. Vol 1. New York: Oxford University Press, 1991:577.
Further reading and resources
- • Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341: 738-46. [DOI] [PubMed] [Google Scholar]
- • Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ 2002;324: 160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Enoch S, Shaaban H, Dunn KW. Informed consent should be obtained from patients to use products (skin substitutes) and dressings containing biological material. J Med Ethics 2005;31(1): 2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Jones J, Nelson E. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2005;(1): CD001737. [DOI] [PubMed]
- • US National Human Genome Research Institute. www.genome.gov