Abstract
Toll-like receptors (TLRs) are primary sensors of both innate and adaptive immune systems and play a pivotal role in response against structurally conserved components of pathogens. Synthetic bacterial lipoprotein (BLP) Pam3Cys-SK4 is a TLR2 agonist that is capable of modulating T cell immune responses. We show here that BLP, together with anti-CD3 antibody [T cell receptor (TcR) activation], induced proliferation of both CD4+CD25+ regulatory T cells (Tregs) and CD4+CD25− (effector) T cells in the absence of antigen-presenting cells. The expanded Tregs showed a transient loss of suppressive activity. Moreover, BLP rendered effectors resistant to the suppression of Tregs by increasing IL-2 secretion. BLP also transiently suppressed the induction of Foxp3 (X-linked forkhead/winged helix transcription factor) mRNA in Tregs at the first 8–15 h after T cell receptor activation. Consistent with this observation, BLP-stimulated Tregs regained their inhibitory activity and prevented spontaneous colitis induced by effectors in severe combined immunodeficient mice. Our results demonstrate a previously unrecognized pathway by which TLR expressed on T cells may directly modulate the immune response. Thus, during an acute bacterial infection, BLP may rapidly increase the host's adaptive immunity by expanding effectors and also by attenuating the suppressive activity of Tregs. In the process, BLP also expands the Tregs, which recover their suppressive activity when the infection has subsided, in time to limit potential autoimmunity that might result from the overactivated effectors.
Keywords: colitis, Pam3CSK, Foxp3, Leishmania
Regulatory T cells have subsumed the role, if not the characteristics, of the much-maligned suppressor T cells. There are at least three major types of regulatory T cells: Th3, Tr1, and CD4+CD25+ T cells, with overlapping functions (1–3). CD4+CD25+ regulatory T cells (Tregs) are arguably the best characterized because of the ease of obtaining a large number of the cells. However, there remains considerable uncertainty in the characteristics, functions, and regulation of these cells. A fundamental question is how a successful protective immunity could take place under the suppression of a range of regulatory T cells. The function of regulatory T cells must be tightly controlled to meet different requirements of immunity. However, the mechanism for the selective control of regulatory T cell functions remains obscure. Because Tregs are naturally occurring, it is anticipated that the cells would be strongly influenced by pathogens in an innate manner (4), including the activation of Toll-like receptors (TLRs).
TLRs play an important role in the induction and regulation of innate and adaptive immunity (4–7). Recent reports demonstrate that MyD88 (a key adaptor protein in most of the TLR signaling pathways) in dendritic cells (DCs) is required for attenuating Treg functions, leading to robust immune responses (8, 9). However, the selective usage of TLRs is unknown. The TLR signal is necessary for the maturation of DCs and initiation of immunity. Mature, but not immature, DCs could abrogate Treg function (10, 11). However, the precise mechanism by which mature DCs modulate Tregs is not fully understood. It has been reported that Tregs could be expanded by LPS, a product of Gram-negative bacteria and a ligand for TLR 4 (12). However, this finding has yet to be independently confirmed. Cytokines, especially IL-2, play a critical role in the modulation of Treg function. A low dose of IL-2 is required for maintaining Treg-mediated suppressive function (13). In contrast, high levels of IL-2 reverse Treg functions (14, 15). However, the cellular source of the high level of IL-2 during an immune activation, and the mechanism by which IL-2 affects Tregs in the immune network, remains unclear.
We have recently demonstrated that TLR2 and TLR4 were both expressed on the surface of CD4+ and CD8+ T cells (16). However, only TLR2 was functionally active on T cell receptor (TcR)-primed T cells and memory T cells. Furthermore, TLR2 signaling acted as a costimulator of T cells and might contribute to the maintenance of T cell memory. We reasoned that bacterial lipoprotein (BLP) Pam3CSK, a TLR2 ligand, might also play a direct role in the functions of Tregs.
Data reported here suggest the following scenario. To combat an acute infection, the host may make use of the BLP produced by pathogens to enhance CD4+CD25− effector cell expansion and inhibit the suppressive effect of Tregs. BLP also induces the effector cells to produce more IL-2, which renders the effector cells refractory to the suppressive effect of Tregs. BLP, together with IL-2, also expands the Tregs, whose suppressive activity recovers when the BLP level has subsided after a successful host response, in time to prevent any potential autoimmune reaction resulting from the expanded effector cell populations. Our results therefore suggest a previously uncharacterized role of TLR2 signaling in regulating the adaptive immune response by acting directly on both effector and regulatory T cells.
Results
CD4+CD25− and CD4+CD25+ T Cells Functionally Expressed Cell Surface TLR2.
CD4+CD25− (effector) and CD4+CD25+ (regulatory) T cells were purified from BALB/c mice by magnetic-activated cell sorting, followed by sorting with a FACSAria cell sorter (BD Biosciences, Franklin Lakes, NJ). The purity was >99.9% (Fig. 1A). Tregs, but not effectors, expressed Foxp3 (X-linked forkhead/winged helix transcription factor) (Fig. 1A). TLR2 was stably expressed intracellularly in both effectors and Tregs, but no cell surface TLR2 was detectable on normal resting T cells. After activation by αCD3, TLR2 was readily detected on the surface of effectors and Tregs (Fig. 1B). In contrast, little or no cell surface TLR4 was detected on either population of cells (data not shown).
Fig. 1.
CD4+CD25− (effector) and CD4+CD25+ (regulatory) T cells functionally expressed TLR2. (A) FACS analysis shows the purity of Tregs, which were Foxp3+. The same degree of purity was achieved for effectors (data not shown), which were Foxp3−. −ve, isotype control. (B) Cells were either freshly isolated or activated for 72 h with plate-bound αCD3. Fluorescence density was measured by gating CD4+ and CD25+ cells. Control was isotype-matched IgG. T cells from TLR2−/− mice stained negative (data not shown). (C) Tregs were labeled with 5-(-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) and cultured with APC+ αCD3 and BLP (5 μg/ml) for 3 days. (D) Tregs and effectors were cultured with αCD3 and BLP for 3 days, and cellular proliferation was determined by [3H]thymidine incorporation. (E) Tregs and effectors from WT or TLR2−/− mice were cultured as in D, and cell proliferation was determined. IL-2 in the supernatant was measured by using ELISA. Data are mean ± SD (n = 3) and are representative of three experiments. ∗∗, P < 0.01 compared with control culture without BLP.
To examine the functional specificity of TLR2 expression on T cells, effectors and Tregs were cultured with αCD3 with or without BLP or LPS. BLP, but not LPS, significantly enhanced the proliferation and cytokine production by both effectors and Tregs (Fig. 7, which is published as supporting information on the PNAS web site). We also tested LPS from different strains of bacteria. Only LPS from Salmonella typhimurium induced a weak response (Fig. 7B). Thus, activated Tregs and effectors functionally express TLR2 but not TLR4.
The enhancing effect of BLP on Treg proliferation was also demonstrated by 5-(-6)-carboxyfluorescein diacetate, succinimidyl ester labeling. BLP markedly increased the cell division of Tregs (Fig. 1C). Effectors and Tregs had a similar dose response to BLP, reaching a plateau at 1–20 μg/ml (Fig. 1D). Effectors and Tregs from TLR2−/− mice failed to respond to BLP (Fig. 1E). The effect of BLP was probably not due to an indirect effect of BLP acting on TLR2 of antigen-presenting cells (APCs), because the deliberate addition of 5% live CD4+ T cell-depleted spleen cells from the WT mice into the culture of αCD3-treated effectors or Tregs from TLR2−/− did not enhance the proliferation of the T cells (Fig. 8, which is published as supporting information on the PNAS web site).
BLP Abrogated the Suppressive Effect of Treg Cells.
We next determined whether BLP has an effect on the suppressive functions of Tregs by using the standard suppression assay. Effectors and Tregs from BALB/c mice were cocultured with sαCD3 and APC with or without BLP. As expected, Tregs profoundly suppressed effector proliferation and IFN-γ production. The suppression was reversed by BLP. However, no reversal was observed in cultures of cells from TLR2−/− mice (Fig. 2A).
Fig. 2.
BLP abrogated the suppressive functions of Treg cells. (A) Tregs and effectors from C57BL/6 WT or TLR2−/− mice were cultured alone or together (1:1 ratio) with sαCD3 and APC from TLR2−/− mice, with or without BLP, for 3 days. Cellular proliferation and IFN-γ synthesis were determined. (B) Tregs and effectors from C57BL/6 WT or TLR2−/− mice were cultured as in A in various combinations as indicated. BLP partially reversed the suppressive effect of Tregs, even when either cell type was nonresponsive to BLP (TLR−/−). Data are mean ± SD (n = 3) and are representative of at least three experiments. ∗∗, P < 0.01 compared with culture without BLP.
To investigate which cell population contributed to the abrogation of suppression, effectors and Tregs were purified from WT and TLR2−/− mice. BLP partially but significantly reversed the suppressive effect of Tregs from TLR2−/− mice on effectors from WT mice (Fig. 2B). Conversely, BLP also partially reversed the suppressive effect of Tregs from WT mice on effectors from TLR2−/− mice. These results demonstrate that BLP acts on both effectors and Tregs in the reversal of the suppressive effect of Tregs.
IL-2 Produced by Effectors Abrogated the Suppressive Effect of Tregs.
We next investigated the mechanism by which BLP reversed the suppressive effect of Tregs. It is known that IL-2 is required for the differentiation of Tregs (15) and that the expanded Tregs become nonsuppressive, the activity of which recovers after a rest period in the absence of IL-2 (15). We therefore examined the role of IL-2 in our culture system.
Tregs and effectors were cultured with sαCD3, APC, and BLP for 3 days. IL-2 was not detectable in cultures without BLP. High concentrations of IL-2 were produced by effectors when BLP was added (Fig. 3A). However, little or no IL-2 was produced by Tregs, APCs, or bone marrow-derived DCs (data not shown). Confirming this, effectors from IL-2−/− mice did not proliferate or produce IFN-γ when cultured with αCD3 and BLP (Fig. 9A, which is published as supporting information on the PNAS web site). Thus, effectors were the only source of IL-2 produced.
Fig. 3.
IL-2 produced by effectors played an important role in the BLP-mediated abrogation of the suppressive effect of Tregs. Effectors and Tregs were cultured separately or together with sαCD3 and APC for 3 days. (A) Only effectors produced appreciable amounts of IL-2 in response to BLP. (B) Tregs and effectors were cultured together with or without BLP and αIL-2 or normal IgG. (C) The proliferation of effectors could be completely suppressed by formalin-fixed Tregs. This suppression was reversed by the addition of IL-2. (D) Supernatant was collected from cultures of effectors stimulated for 3 days with BLP. Supernatant from WT, but not TLR2−/−, mice reversed the suppressive effect of Tregs. The reversal was neutralized by αIL-2. (E) Effectors were cocultured with Tregs in the presence of the supernatant (1 or 1/5 dilution) of effectors stimulated with or without (con) BLP. (F) Tregs were cultured with supernatants from effectors (of WT or IL-2−/− mice) stimulated with or without (con) BLP. Tregs proliferated significantly when cultured with BLP alone or with the supernatant of effectors from WT, but not IL-2−/−, mice. Data are mean ± SD (n = 3–4) and are representative of at least three experiments. ∗∗, P < 0.01 compared with controls.
To test the effect of IL-2 produced by the effectors, Tregs and effectors were cocultured with BLP with or without αIL-2 antibody. As expected, BLP abrogated the suppression in the coculture. The effect of BLP was partially reversed by αIL-2 (Fig. 3B). αIL-2 also blocked the enhancing effect of BLP on effector proliferation. Thus, IL-2 secreted from BLP-activated effectors contributed significantly to the BLP-mediated abrogation of the suppressive effect of Tregs. Confirming the importance of IL-2, BLP only partially reversed the inhibitory effect of Tregs when cultured with effectors from IL-2−/− mice (Fig. 9B).
We then investigated which cell population was affected by IL-2. Tregs were activated by αCD3 for 3 days, rested with IL-2 for another 3 days, and then fixed by formaldehyde. Fixed Tregs suppressed the proliferation of naïve effectors. However, the suppression was completely reversed by the addition of IL-2 (Fig. 3C). In this experiment, IL-2 could not have affected the fixed Tregs and therefore played a direct role in the activation of effectors, rendering them refractory to the suppressive effect of Tregs.
To confirm the importance of IL-2 produced by effectors, Tregs from C57BL/6 WT, TLR2−/−, or IL-2−/− mice were activated with BLP. Supernatant of BLP-activated effectors from WT mice abrogated the suppressive effect of Tregs in a dose-dependent manner (Fig. 3 D and E). This suppression was reversed by the presence of αIL-2 (Fig. 3D). In contrast, supernatants of effectors from TLR2−/− (Fig. 3D) or IL-2−/− (Fig. 3E) mice had no effect on the suppressive effect of Tregs. Supernatant from effectors of WT, but not IL-2−/−, mice markedly expanded the proliferation of Tregs (Fig. 3F), showing that the proliferation of Tregs was due to IL-2 produced by the effectors and not any residual BLP in the culture supernatants.
IL-6 was reported to abrogate the suppressive effect of Tregs in a DC-dependent manner (8, 10, 11). We then investigated the potential role of IL-6. Effectors, but not Tregs, produced IL-6 when activated by αCD3 plus BLP (data not shown). The suppressive effect of Tregs was not affected by a neutralizing αIL-6 antibody (Fig. 10A, which is published as supporting information on the PNAS web site). Furthermore, BLP similarly reversed the suppressive effect of Tregs cultured with effectors from WT or IL-6−/− mice (Fig. 10 B and C). Thus, IL-6 was not involved in the BLP-mediated abrogation of suppression of effectors by Tregs.
BLP-Stimulated Treg Cells Regained Their Suppressive Function.
We then determined whether the loss of the suppressive effect of the BLP-expanded Tregs was transient. Tregs were cultured with αCD3 and BLP for 15 h, washed, and then cocultured with freshly purified effectors. As expected, the Tregs lost their suppressive activity (Fig. 4A). However, when the Tregs were cultured for 3 days with BLP and rested for a further 3 days in IL-2, they regained their suppressive function (Fig. 4A).
Fig. 4.
BLP treatment transiently abrogated the suppressive activity and reduced the expression of Foxp3 of Tregs. (A) Effector and Tregs from BALB/c mice were cultured alone or together for 3 days with sαCD3 and APC. In the cocultures, Tregs were preactivated with αCD3 and BLP for 15 h or for 3 days and rested for another 3 days before being cocultured with freshly isolated effectors (7 days). BLP-treated Tregs regained their suppressive activity after the rest period. Data are mean ± SD (n = 3) and are representative of three experiments. ∗∗, P < 0.01 compared with effectors alone. (B and C) Tregs and effectors from BALB/c (B) or TLR2−/− and WT C57BL/6 (C) mice were activated by αCD3 with or without BLP. Cells were analyzed for Foxp3 mRNA expression by real-time PCR. TcR triggering markedly elevated Foxp3 expression at 8–15 h. BLP significantly suppressed Foxp3 expression in the Tregs from WT, but not TLR2−/−, mice. Results are presented as percentage of hypoxanthine phosphoribosyltransferase expression and are representative of at least three experiments. ∗∗, P < 0.01 compared with nonactivated cells. Attempts to demonstrate the effects of BLP on Foxp3 expression by using intracellular staining were not successful, probably because of the lower sensitivity of flow cytometry (data not shown).
BLP Transiently Suppresses the Expression of Foxp3.
Foxp3 is closely associated with the suppressive function of Tregs (17–20). Foxp3 is constitutively expressed in Tregs at a level not known to be affected by Treg activation. However, thus far, all of the studies examined the Foxp3 expression at least 24 h after Treg activation. We reasoned that because the effect of BLP occurred before 15 h after Treg activation, the Foxp3 expression might also be affected by BLP at time points earlier than 24 h. Tregs and effectors were activated with αCD3 with or without BLP. Relatively high levels of Foxp3 detected in Tregs at 0 h were markedly enhanced by αCD3 activation, peaking at 8–15 h and returning to the resting level by 24 h (Fig. 4B). In contrast, Tregs activated in the presence of BLP did not show elevated Foxp3 expression. Instead, Foxp3 expression of BLP-treated Tregs was significantly reduced below that of the constitutive level 8 h after activation before returning to the resting level. No Foxp3 expression was detected in effectors. We then tested the specificity of the effect of BLP. Although Foxp3 expression of WT Tregs was decreased by BLP at 8 h after activation, Foxp3 expression of TLR2−/− Tregs was not affected by BLP (Fig. 4C). Thus, BLP transiently down-regulated the Foxp3 expression of activated Tregs, which may account for the direct transient abrogation of the suppressive functions of Tregs.
Effects of BLP-Stimulated Tregs in Vivo.
Tregs are highly effective in treating an established colitis in effector-reconstituted severe combined immunodeficient (SCID) mice (21, 22). We then investigated whether BLP affected Treg function in vivo. SCID mice were reconstituted with effectors and infected with Leishmania major (22, 23). Mice were injected i.p. 21 days later with Tregs activated with αCD3 and BLP for 15 h. Untreated SCID mice developed severe colitis from day 14, whereas those given Tregs were cured by day 50. BLP-treated Tregs showed a significantly delayed therapeutic effect, although all of the mice eventually recovered from the disease (Fig. 5 A and B). This recovery was reflected by the suppression of IFN-γ and IL-4 but elevation of IL-10 production (Fig. 5C). Effector-reconstituted SCID mice contained L. major infection, and the protection was reversed by the Tregs (22). However, BLP-treated Tregs failed to reverse the protective effect of effectors (Fig. 5D). The number and viability of the effectors and Tregs used for cell transfer were comparable (data not shown). These results are consistent with the notion that the initial loss of suppressive activity of BLP-treated Tregs allowed the effector cells to eliminate the pathogen and that the Tregs recovered in time to subsequently control chronic inflammatory colitis.
Fig. 5.
BLP-stimulated Tregs recovered their suppressive functions in vivo. SCID mice were adoptively transferred i.p. with 5 × 105 effectors and infected with L. major in the footpad. The diseased mice were injected i.p. (on day 21) with PBS (CD25−/PBS), 5 × 105 Tregs (CD25−/CD25+), or BLP-activated Tregs for 15 h (αCD3 plus BLP). (A and B) Mice were monitored for body weight (A) and histology of the colon (B) at the end of experiment (8 weeks). Mesenteric lymph node cells were collected and cultured in vitro with αCD3. (C) Cytokine productions were determined by using ELISA. (D) Leishmania infection was also determined as the difference in the thickness between the infected right footpad and the uninfected left footpad. The footpad lesions parallel the parasite load in the footpad (ref. 23 and data not shown). Data are mean ± SD (n = 6) and are representative of two experiments.
Discussion
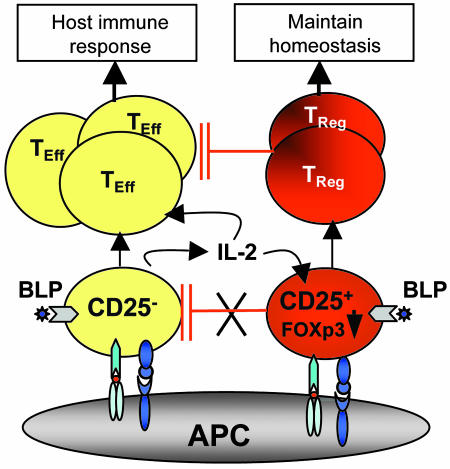
We demonstrate here that TLR2 signaling in T cells has distinct effects on effectors and Tregs. TLR2 signaling alone had little or no effect on T cell functions. However, TLR2 agonist strongly enhanced the proliferation of TcR-triggered Tregs, rendering them transiently nonsuppressive. BLP also enhanced the proliferation and cytokine (especially IL-2) production of TcR-activated effectors. IL-2 thus produced could further expand the effectors and the Tregs, but renders the effector cells refractory to the suppressive effect of Tregs. Thus, TLR2 signaling in T cells represents a unique mechanism for regulating the host's immune response against pathogens. The mechanism and biological implication of TLR2 signaling in T cells is schematically represented in Fig. 6.
Fig. 6.
Schematic representation of the role of BLP in T cell homeostasis. Mammalian hosts could use BLP produced by pathogens to enhance the proliferation of, and IL-2 production by, CD4+CD25− effector (TEff) cells to combat the pathogens. BLP also transiently switches off Tregs (probably by down-regulating Foxp3) to boost the effectors. IL-2 produced by TEff could also transiently switch off the Treg function. Both BLP and IL-2 could also expand Tregs. When the level of BLP is reduced after elimination of the pathogens, the expanded Tregs could regain their suppressive function to prevent potential autoimmunity that might result from the expanded TEff cells.
It is intriguing that TLR2, but not TLR4, is functionally expressed on T cells. This finding is consistent with the vast majority of the literature, which shows that T cells are generally not responsive to LPS (24–27). We have earlier shown that although activated human CD4+ T cells expressed cell surface TLR4, these cells failed to respond to LPS (16). LPS (from S. typhimurium) has recently been reported to activate CD4+CD25+ T cells in mice (12). The discrepancy of our results and this earlier report remains to be resolved. It could well be due to the different culture conditions used. The potential contamination of the LPS preparation with other agonists could not be excluded.
TLR message is constitutively expressed in all T cells (12, 28, 29). However, the cell surface expression of TLR proteins requires TcR activation. We reported earlier that TLR2 is functionally expressed on human and mouse CD4+ and also CD8+ T cells (16). This finding was later extended to TLR5 (30) and TLR8 (31). TLR5 activation by bacterial flagellin was reported to increase Treg proliferation and suppressive activity by means of enhanced Foxp3 expression (30), whereas TLR8 activation by type-D CpG oligonucleotides reversed the suppressive function of Tregs (31). The mechanism by which the transportation of TLR2 from the cytoplasm to the cell membrane initiated by TcR triggering is unclear. This process provides a safeguard to prevent constant activation and expansion of T cells by TLR2 agonists, which are highly conserved among pathogens.
LPS-induced IL-6 produced by DCs was reported to be involved in the reversal of the suppressive effect of Tregs (8, 9, 32). In our study, focusing on a direct effect of BLP on T cells, IL-6 did not appear to be involved. In contrast, IL-2 clearly plays a pivotal role in the BLP-mediated abrogation of Treg function. IL-2 produced by effectors could influence the Treg function in two ways: (i) IL-2 induces T effector cell proliferation, rendering them refractory to the suppression of Tregs. (ii) IL-2 induces Treg proliferation and transiently switches off their suppressive activity, which could be restored after a rest period (15). The mechanism by which IL-2 abrogates the suppressive function of Tregs is currently unclear, but is unlikely to involve Foxp3, because IL-2 did not affect the expression of Foxp3 in Tregs (data not shown).
Tregs could also be switched off by a direct action of BLP. The transient loss of suppressive function of Tregs was not due to BLP modulating CTLA-4 (cytotoxic T lymphocyte antigen 4) or glucocorticoid-induced TNF receptor expression (data not shown) but was correlated with the down-regulation of Foxp3 expression. Foxp3 expression in Tregs could be up-regulated at the early stage of TcR triggering. This elevated Foxp3 expression may be associated with Treg function. The presence of BLP suppressed the Foxp3 expression at this early stage of TcR activation. Formal proof of a direct involvement of Foxp3 in BLP-mediated abrogation of Tregs must be explored.
The in vivo results obtained by using the Leishmania/colitis model in SCID mice are consistent with the interpretation of our in vitro data. In contrast to untreated Tregs, BLP-treated Tregs were unable to reverse the protective function of effectors against L. major infection. However, the BLP-treated Tregs were able to contain colitis, albeit with a significant delay in onset compared with untreated Tregs. It thus appears that BLP induced a transient loss of the suppressive activity of Tregs and enabled the effector cells to eliminate the parasite. The subsequent recovery of suppressive activity of the BLP-treated Tregs was able to prevent the subsequent chronic inflammatory colitis (Fig. 6). It is likely that this finding may reveal a common pathway by which some other TLRs (30, 31) may have comparable functions in regulating the immune functions against distinct pathogens.
Materials and Methods
Mice.
BALB/c, C57BL/6, and SCID mice (C.B-17) of the BALB/c background were purchased from Harlan Olac (Bicester, U.K.). TLR2−/− mice of the C57BL/6 background (originally from Shizou Akira, University of Osaka, Osaka) were kindly provided by Jean Langhorne (National Institute for Medical Research, London). IL-2−/− mice of the C57BL/6 background were kindly provided by Fiona Powrie (University of Oxford, Oxford). IL-6−/− mice of the BALB/c background were bred and housed under pathogen-free conditions in the Biological Service Central Facilities at University of Glasgow according to the United Kingdom Home Office guidelines. Both male and female mice were used at 6–12 weeks of age.
Purification of T Cell Subsets.
CD4+ T cells were purified from lymph nodes by negative selection by using magnetic beads (AutoMACs; Miltenyi Biotech, Auburn, CA) and then sorted into CD4+CD25+ or CD4+CD25− populations with a FACSAria cell sorter (BD Biosciences) by using phycoerythrin-anti-CD25 antibody (BD Biosciences). The purity of cells was >99.9%.
T Cell Cultures.
T cells were cultured in RPMI medium 1640 supplemented with 10% heat-inactivated FCS (Harlan)/2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin/0.05 M 2-mercaptoethanol (complete medium). Cultures were incubated at 37οC in 5% CO2. Cells (2.5 × 104 cells per 200-μl well) were activated by plate-bound αCD3 antibody (5 μg/ml; R & D Systems) in a flat-bottom 96-well plate (Nunc), and proliferation assays were performed in triplicate for up to 3 days before the incorporation of [3H]thymidine in the last 8–12 h of culture. In some experiments, IL-2 (10 ng/ml; GlaxoSmithKline), anti-IL-2 (10 μg/ml; R & D Systems), or anti-IL-6 (1 μg/ml; R & D Systems) antibodies were also added in the cultures.
For suppression assays, effectors (2.5 × 104 cells per 200-μl well) were cultured in the presence of soluble anti-CD3 antibody (sαCD3) (1 μg/ml) with equal numbers of APCs. Tregs were also added in selected wells. In some experiments, BLP (Pam3CSK; EMC Microcollection, Tuebingen, Germany) or LPS (derived from Salmonella enteritdiis, Salmonella minnesota, or S. typhimurium, all from Sigma) were added at the beginning of the culture. In some experiments, cells were fixed with 1% paraformaldehyde in PBS by gently adding the live cells into the fixing buffer. Cells were fixed for 1.5 h on ice with gentle agitation, washed three to four times, and resuspended in the complete medium. For cell division analysis, Tregs were labeled with 5-(-6)-carboxyfluorescein diacetate, succinimidyl ester (Molecular Probes), cultured for 3 days, and stained with APC-anti-CD4 and phycoerythrin-anti-CD25, and fluorescence density was analyzed by flow cytometry by gating double-positive T cell populations.
Flow Cytometry.
Purified T cells were stained with directly conjugated antibodies: αCD4 (BD Biosciences), αCD25 (BD Biosciences), αTLR2 (eBioscience, San Diego), or αTLR4 (produced in our laboratory) with appropriate normal isotype-matched control IgG (BD Biosciences). All samples were preincubated for 20 min with CD16/32 antibody (BD Biosciences) to block Fc receptor. For intracellular staining, the cells were fixed with 2% formaldehyde and washed with Perm/wash solution (FACS buffer plus 0.5% saponin). The cells were analyzed on a FACSCalibur flow cytometer.
Quantitative PCR.
Quantitative PCR was carried out as described in ref. 22. The primers and probes used were as follows: Foxp3, forward 5′-TTTACTCGCATGT TCGCCTACTT-3′, reverse 5′-CTCAAATTCATCTACGGTCCACACT-3′, probe 5′-ACCTGGAAGAATGCCATCCGCCA-3′; hypoxanthine phosphoribosyltransferase, forward 5′-GCAGTACAG CCCCAAAATGG-3′, reverse 5′-AACAAAGTCTGGCCTGTATC CAA-3′, probe 5′-TAAGGTTGCAAGCTTGCTG GTGAA AAGGA-3′.
T Cell Transfer Model of Colitis.
SCID mice were infected in the hind footpad with 1 × 106 stationary phase L. major (LV39) promastigotes 1 day after receiving i.p. 5 × 105 effectors (22, 23). Maintenance of parasite, infection, and measurement of disease progression were as described in ref. 22. Tregs were activated with αCD3 and BLP (5 μg/ml) for 15 h and injected i.p. (5 × 105 cells) into SCID mice on day 21. Mice were monitored for body weight at regular intervals. At the end of the experiment, draining lymph node cells were harvested and cultured (8 × 105 cells per ml) in vitro with αCD3. Culture supernatant (72 h) was assayed for cytokines by ELISA by using paired antibodies (BD Pharmingen).
Histological Examination.
Colons were removed and fixed in 10% buffered formalin. Paraffin-embedded sections (6 μm) were stained with hematoxylin/eosin and scored (22). The sections were scanned with a Duoscan T2000XL microscope (Agfa-Gevaert N.Y., Belgium), and pictures were taken with a Fuji X digital camera (HC-300Z) at ×40 magnification.
Statistical Analysis.
Statistics were analyzed by using minitab software (Minitab, State College, PA) for the PC. The analyses were performed by using Student's t test. All data are representative of at least three independent experiments.
Supplementary Material
Acknowledgments
We thank Dr. Jean Langhorne for providing the TLR2−/− mice originally constructed by Dr. Shizou Akira, Prof. Fiona Powrie for providing the IL-2−/− mice, Mr. Rod Ferrier (University of Glasgow) for assistance in histology preparation, and Barbara Young for photography. This work was supported by the Medical Research Council, the Wellcome Trust, the European Commission, and the Chief Scientist's Office of Scotland.
Abbreviations
- APC
antigen-presenting cell
- BLP
bacterial lipoprotein
- Foxp3
X-linked forkhead/winged helix transcription factor
- TcR
T cell receptor
- TLR
Toll-like receptor
- Treg
CD4+CD25+ regulatory T cell
- SCID
severe combined immunodeficient
- DC
dendritic cell
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J. E., Roncarolo M. G. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 5.Janeway C. A., Jr., Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K., Kaisho T., Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 7.Pasare C., Medzhitov R. Adv. Exp. Med. Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Pasare C., Medzhitov R. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 9.Pasare C., Medzhitov R. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki S., Iyoda T., Tarbell K., Olson K., Velinzon K., Inaba K., Steinman R. M. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo T., Hatton R. D., Oliver J., Liu X., Elson C. O., Weaver C. T. J. Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 12.Caramalho I., Lopes-Carvalho T., Ostler D., Zelenay S., Haury M., Demengeot J. J. Exp. Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton A. M., Donovan E. E., Piccirillo C. A., Shevach E. M. J. Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T. W., Sakaguchi S. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton A. M., Shevach E. M. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komai-Koma M., Jones L., Ogg G. S., Xu D., Liew F. Y. Proc. Natl. Acad. Sci. USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hori S., Nomura T., Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 18.Fontenot J. D., Gavin M. A., Rudensky A. Y. Nat. Immunol. 2003;4:330–336. [PubMed] [Google Scholar]
- 19.Khattri R., Cox T., Yasayko S. A., Ramsdell F. Nat. Immunol. 2003;4:337–342. [PubMed] [Google Scholar]
- 20.Yagi H., Nomura T., Nakamura K., Yamazaki S., Kitawaki T., Hori S., Maeda M., Onodera M., Uchiyama T., Fujii S., Sakaguchi S. Int. Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 21.Mottet C., Uhlig H. H., Powrie F. J. Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Hu B., Xu D., Liew F. Y. J. Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 23.Xu D., Liu H., Komai-Koma M., Campbell C., McSharry C., Alexander J., Liew F. Y. J. Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 24.Tough D. F., Sun S., Sprent J. J. Exp. Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 26.Castro A., Bemer V., Nobrega A., Coutinho A., Truffa-Bachi P. Eur. J. Immunol. 1998;28:488–495. doi: 10.1002/(SICI)1521-4141(199802)28:02<488::AID-IMMU488>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Netea M. G., Sutmuller R., Hermann C., Van der Graaf C. A., Van der Meer J. W., van Krieken J. H., Hartung T., Adema G., Kullberg B. J. J. Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M., Bosisio D., Polentarutti N., D’Amico G., Stoppacciaro A., Mancinelli R., van’t Veer C., Penton-Rol G., Ruco L. P., Allavena P., Mantovani A. J. Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 29.Zarember K. A., Godowski P. J. J. Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 30.Crellin N. K., Garcia R. V., Hadisfar O., Allan S. E., Steiner T. S., Levings M. K. J. Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 31.Peng G., Guo Z., Kiniwa Y., Voo K. S., Peng W., Fu T., Wang D. Y., Li Y., Wang H. Y., Wang R. F. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 32.Fehervari Z., Sakaguchi S. Int. Immunol. 2004;16:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.