Abstract
Acetylcholinesterase (AChE) activity has traditionally been monitored as a biomarker of organophosphate (OP) and/or carbamate exposure. However, AChE activity may not be the most sensitive endpoint for these agrochemicals, because OPs can cause adverse physiological effects at concentrations that do not affect AChE activity. Carboxylesterases are a related family of enzymes that have higher affinity than AChE for some OPs and carbamates and may be more sensitive indicators of environmental exposure to these pesticides. In this study, carboxylesterase and AChE activity, cytochrome P4501A (CYP1A) protein levels, and mortality were measured in individual juvenile Chinook salmon (Oncorhynchus tshawytscha) following exposure to an OP (chlorpyrifos) and a pyrethroid (esfenvalerate). As expected, high doses of chlorpyrifos and esfenvalerate were acutely toxic, with nominal concentrations (100 and 1 μg/l, respectively) causing 100% mortality within 96 h. Exposure to chlorpyrifos at a high dose (7.3 μg/l), but not a low dose (1.2 μg/l), significantly inhibited AChE activity in both brain and muscle tissue (85% and 92% inhibition, respectively), while esfenvalerate exposure had no effect. In contrast, liver carboxylesterase activity was significantly inhibited at both the low and high chlorpyrifos dose exposure (56% and 79% inhibition, respectively), while esfenvalerate exposure still had little effect. The inhibition of carboxylesterase activity at levels of chlorpyrifos that did not affect AChE activity suggests that some salmon carboxylesterase isozymes may be more sensitive than AChE to inhibition by OPs. CYP1A protein levels were ∼30% suppressed by chlorpyrifos exposure at the high dose, but esfenvalerate had no effect. Three teleost species, Chinook salmon, medaka (Oryzias latipes) and Sacramento splittail (Pogonichthys macrolepidotus), were examined for their ability to hydrolyze a series of pyrethroid surrogate substrates and in all cases hydrolysis activity was undetectable. Together these data suggest that (1) carboxylesterase activity inhibition may be a more sensitive biomarker for OP exposure than AChE activity, (2) neither AChE nor carboxylesterase activity are biomarkers for pyrethroid exposure, (3) CYP1A protein is not a sensitive marker for these agrochemicals and (4) slow hydrolysis rates may be partly responsible for acute pyrethroid toxicity in fish.
Keywords: Pyrethroid, Organophosphate, Chinook salmon, Carboxylesterase, Acetylcholinesterase, Cytochrome P4501A
1. Introduction
Agrochemical usage practices are currently shifting, with a general movement away from organophosphates (OPs) towards pyrethroid pesticides (Casida and Quistad, 1998). The ecological implications of this large-scale shift in pesticide application are unknown. Pyrethroids generally have low mammalian toxicity (Abernathy and Casida, 1973; Casida et al., 1983; Casida and Quistad, 1995), especially compared to many OP pesticides. However, there have been several reports regarding the sensitivity of aquatic invertebrates and some fish species to pyrethroids (Bradbury and Coats, 1989a; Werner et al., 2002; Denton et al., 2003). There is subsequently concern that the ecological consequences of increased pyrethroid application on aquatic ecosystems could be far-reaching.
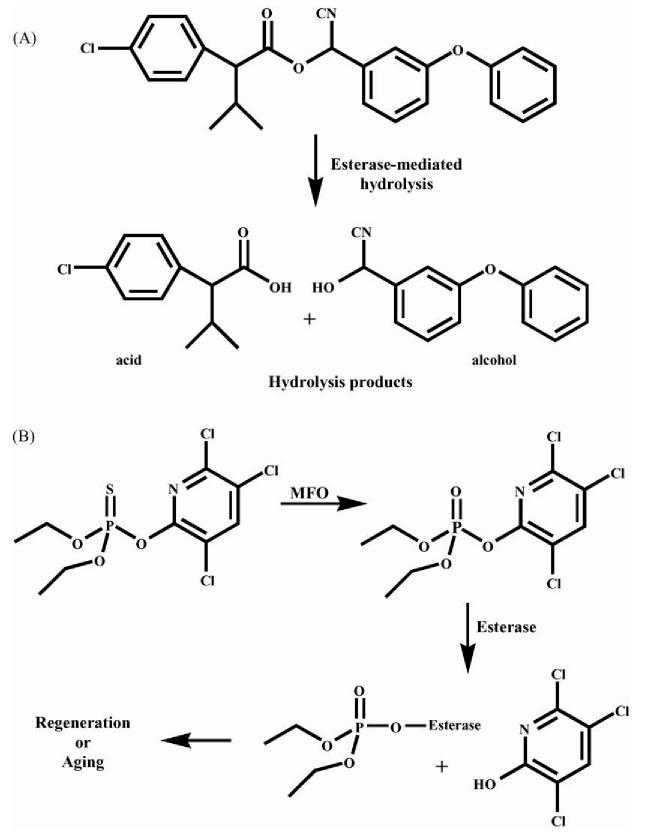
Carboxylesterases are a class of enzymes that hydrolyze ester-containing compounds to the corresponding alcohol and acid (hydrolysis products) (Satoh and Hosokawa, 1998; Wheelock et al., 2005). These enzymes are important in the metabolism and subsequent detoxification of many xenobiotic and endogenous compounds, including pyrethroids and OPs (Fig. 1). Carboxylesterases reduce pyrethroid toxicity by hydrolyzing these compounds to less toxic metabolites (Abernathy and Casida, 1973; Wheelock et al., 2004). OPs are generally not esterase substrates; however they bind stoichiometrically to both carboxylesterases and acetylcholinesterases (AChE) (Sogorb and Vilanova, 2002; Casida and Quistad, 2004). Carboxylesterases have an increased affinity over AChE for some OPs and it has been suggested that carboxylesterases act as a “sink” for OPs, thus protecting the organism against OP toxicity (Maxwell, 1992). Carboxylesterase activity can therefore serve as a detoxification route for both pyrethroids and OPs (Sogorb and Vilanova, 2002). Conversely, simultaneous exposure to OPs and pyrethroids causes synergistic toxicity through OP-induced esterase inhibition rendering the enzyme unable to hydrolyze and thus detoxify pyrethroids (Gaughan et al., 1980; Denton et al., 2003).
Fig. 1.
Esterase detoxification mechanisms. (A) The pyrethroid esfenvalerate is hydrolyzed by esterase to the corresponding acid and alcohol. This process reduces the toxicity of the pyrethroid. (B) The organophosphate chlorpyrifos is converted by mixed-function oxidases (MFO) to the active oxon form, which in turn inhibits esterase. After inhibition, the enzyme can go through two different pathways; regeneration, where the enzyme regains catalytic activity, or aging, in which catalytic activity is lost.
Mixed-function oxidases (MFOs), including the cytochrome P450 monooxygenases (CYP), comprise a superfamily of enzymes that can interact with both OPs and pyrethroids (Casida and Quistad, 1995; Poet et al., 2003). CYP-mediated OP metabolism converts the thione (P S) to the corresponding oxon (P O) (Fukuto, 1990), which is the form that inhibits a number of enzymes including AChE (Fulton and Key, 2001), carboxylesterase (Maxwell, 1992; Casida and Quistad, 2004) and the CYPs themselves (Tang et al., 2002). For example, CYP-mediated desulfuration of chlorpyrifos in rats produces chlorpyrifos-oxon (Fukuto, 1990), in the process releasing the sulfur ion which can then suppress CYP activity through binding to the heme group (Tang et al., 2002). OPs also suppress CYP activity in fish, including the widely used pollutant biomarker enzyme, CYP1A (Flammarion et al., 1998). In contrast to OPs, in which CYP-mediated metabolism produces toxic as well as nontoxic metabolites, CYP metabolism of pyrethroids is exclusively a detoxification process (Casida and Quistad, 1995). Elevated CYP activity is an important mechanism for insect resistance to pyrethroids, including esfenvalerate (Scott, 1999). Unlike OPs, which have been reported to suppress CYPs, pyrethroids such as esfenvalerate can have variable effects, altering some CYP isoforms (Barry et al., 1995), but not others (Barry et al., 1995; Heder et al., 2001).
Some species of fish are very sensitive to pyrethroid toxicity (Bradbury and Coats, 1989b); and it is thought that slow metabolism of the parent compound is partly responsible (Denton et al., 2003). However, few studies have examined pyrethroid metabolism in piscine species and these reports have generally relied upon esterase measurements made on tissue pools, rather than individual fish (Glickman and Lech, 1981; Glickman et al., 1982). While useful, these data only provide information on the average enzyme activity in a population or species and do not indicate the activity range amongst individuals. Data derived from measurements in individuals are important to determine if some individuals metabolize pesticides slower than others, which could potentially correlate with increased sensitivity to pyrethroid or OP exposure. To evaluate inter-individual variations in carboxylesterase and AChE activity, as well as CYP1A protein levels, we evaluated these enzymes in tissue homogenates from individual fish exposed to esfenvalerate or chlorpyrifos. We further investigated esterase activity by measuring pyrethroid hydrolysis in three fish species to compare inter-species variability in activity and to evaluate the relationship between esterase activity level and pesticide toxicity. We hypothesized that fish with lower levels of esterase activity would be more sensitive to pyrethroid and OP toxicity. Results from this work will be valuable in understanding the mechanism of toxicity of two major classes of agrochemicals on multiple fish species. Additionally, these data will be useful for interpreting the impact of increased pyrethroid usage upon aquatic ecosystems.
2. Materials and methods
2.1. Chemicals and equipment
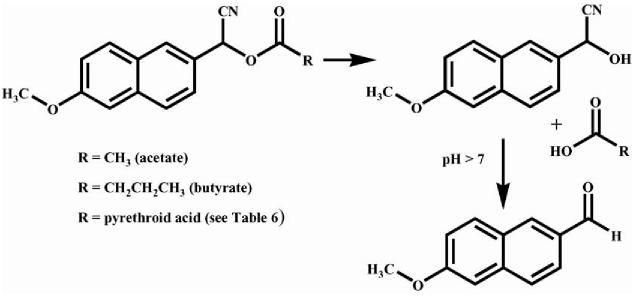
All chemicals and reagents were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO), Invitrogen (Carlsbad, CA), or Fisher Scientific (Pittsburg, PA) unless otherwise stated. Pesticide standards were purchased from Chem Service, Inc. (West Chester, PA). Porcine esterase was purchased from Sigma Chemical Co. (catalog no. E-2884, lot no. 102K7062, 184 U/ml, 10 mg/ml). The following esterase substrates were previously synthesized in our laboratory (Shan and Hammock, 2001; Wheelock et al., 2003; Stok et al., 2004): α-cyano(6-methoxy-2-naphthyl)methyl acetate (see Fig. 2), α-cyano(6-methoxy-2-naphthyl)methyl butyrate (see Fig. 2), (R/S)-α-cyano(6-methoxy-2-naphthyl)methyl-(S)-(+)-2-(4-chlorophenyl)-3-methyl butanoate (αR/S)(2S)—compound 1, (R/S)-α-cyano (6-methoxy-2-naphthyl)methyl-(R)-()-2-(4-chlorophenyl)-3-methyl butanoate (αR/S)(2S)—compound 2, (R/S)-α-cyano(6-methoxy-2-naphthyl)methyl-(R/S)-cis/trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopro-pane carboxylate—compound 3, (R/S)-α–cyano(6-methoxy-2-naphthyl)methyl (cis/trans)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylate— compound 4, (R/S)-α-cyano-(6-methoxy-2-naphthyl) methyl (trans)-3-(2-chloro-2-trifluoromethyl vinyl)-2,2-dimethylcyclopropane carboxylate—compound 5, (R/S)-α-cyano-(6-methoxy-2-naphthyl)methyl (cis)-3-(2-chloro-2-trifluoromethyl vinyl)-2,2-dimethylcyclopropane carboxylate—compound 6, (R/S)-α-cyano-(6-methoxy-2-naphthyl)methyl (cis/trans)-3-(2,2-dimethylvinyl)-2,2-dimethylcyclopropane carboxylate—compound 7, (R/S)-α-cyan(6-methoxy-2-naphthyl)methyl 2,2,3,3-tetramethylcyclopropane carboxylate—compound 8. The monoclonal antibody made against scup CYP1A protein, MAb 1-12-3, was a generous gift of Dr. John Stegeman (Woods Hole Oceanographic Institution).
Fig. 2.
Hydrolysis mechanism for the fluorescent carboxylesterase substrates used in this study. The substrates α-cyano(6-methoxy-2-naphthyl)methyl acetate (acetate) and α-cyano(6-methoxy-2-naphthyl)methyl butyrate (butyrate) are shown here, while the remaining pyrethroid surrogates are shown in Table 6. The α-cyano ester is hydrolyzed to produce the corresponding acid and the cyanohydrin, which spontaneously rearranges to the fluorescent aldehyde at neutral and basic pH. The other carboxylesterase substrate used in this study was p-nitrophenyl acetate (PNPA), which has an acetate moiety coupled to p-nitrophenol and produces the yellow p-nitrophenolate anion and acetic acid upon hydrolysis.
2.2. Study species
Four- to five-month-old Chinook salmon (Oncorhynchus tshawytscha) were supplied by the Nimbus Salmon and Steelhead Hatchery (California Department of Fish and Game, Rancho Cordova, CA) and maintained at the Center for Aquatic Biology and Aquaculture at the University of California Davis according to University of California Davis animal use protocols. Fish were acclimatized under flow-through conditions (15 l/min) for 14 days in a covered,4 ft tall circular FRP-tank (fiber reinforced plastic) with degassed, reoxygenated well water adjusted to 15.2 ± 0.2 °C. Fish were fed a daily ration of soft-moist salmon diet (3/32 in. pellet, Rangen Inc., Buhl, ID).
Medaka (Oryzias latipes) and Sacramento split-tail (Pogonichthys macrolepidotus) were obtained from the University of California Davis Aquatic Toxicology Laboratory and were approximately 10 and 12 months old at the time of sacrifice, respectively. Fish were reared in reconstituted water prepared according to U.S. EPA guidelines (U.S. EPA, 1985). Fish were housed in a partially closed recirculating plexiglass aquarium system equipped with water pump, flow meter, biologic sand filter, particle filter, activated charcoal filter, and ultraviolet light sterilizer. Water in the recirculating system was maintained at 80–100 mg/l CaCO3 (hardness), pH 7.5 ± 2, dissolved O2 7.0 1.0 mg/l, electrical conductivity 300–400 mmho/cm,± alkalinity 30–50 mg/l and 25.0 ± 2.0 °C. Ammonia, nitrate and nitrite were kept below detectable levels by changing charcoal filters weekly and replacing 20% of system water three times a week. Fish were fed a purified casein-based diet (DeKoven et al., 1992).
2.3. Experimental design
Chlorpyrifos (99.5%) and esfenvalerate (Asana™, 98%) stock solutions were prepared in HPLC grade methanol and used immediately. Nominal treatment levels were prepared by diluting stock solutions into individual exposure containers. Two water samples were taken from each treatment, one immediately after spiking the exposure container (0 h) and one just before water renewal (24 h). Water samples were preserved on ice and filtered through baked 0.7 μm glass fiber filters within 24 h (Advantec MFS, Inc., Pleasanton, CA). Terbuthylazine was added as a surrogate and the samples were extracted using C8 solid-phase extraction cartridges (Varian Bond-Elut, 500 mg, 300 cm3 barrel; Varian Inc., Walnut Creek, CA). The cartridges were dried using a syringe to repeatedly force air through each cartridge and stored frozen until analysis. Once removed from storage each cartridge was eluted with 9 ml of ethyl acetate and levels of chlorpyrifos were analyzed by gas chromatography/mass spectrometry (Saturn 2000 GC/MS ion trap system, Varian, Inc.) at the U.S. Geological Survey California District Laboratory (Sacramento, CA) according to published methods (Crepeau et al., 2000).
Juvenile Chinook salmon were exposed to a range of concentrations of chlorpyrifos (1.0, 10 and 100 μg/l) and esfenvalerate (0.01, 0.1 and 1 μg/l) for 96 h. The light:dark ratio was 16 h:8 h. Solvent control fish received the highest concentration of methanol used (50 μl MeOH/l, 0.005% final concentration). Fish (10 per treatment) were exposed individually in 4.0 l, clear soda-lime flint glass containers (Wheaton “800” Redi-Pak* Standard Wide Mouths, Fisher Scientific), each filled with 2 l of test solution. All exposure containers were aerated (∼60 bubbles/min; Tetra DW96-2 Tetra Tech Air Pump, Tetra GmbH; Melle, Germany) and 75% of the test solution was replaced every 24 h. Water temperature was maintained at 14.8 ± 0.5 °C and water quality was monitored daily for pH (8.4 0.2), dissolved oxygen (9.1 ± 0.8 mg/l) and electric conductivity (680 ± 50 μS/cm). Free ammonia was evaluated colorimetrically from three containers each day of the exposure using commercially available kits (EM Science, Gibbstown, NJ). Fish were not fed on the day preceding initiation of the experiment or during the length of the exposure. At the end of the 96 h exposure period, surviving fish were sacrificed by decapitation, dissected and tissue samples flash-frozen in liquid nitrogen and stored at -80 °C. Ten randomly sampled fish from the initial flow-through system were sacrificed and dissected the day before experiment initiation to serve as an unexposed control group.
2.4. Acetylcholinesterase assays
Salmon brains were removed entirely, whereas muscle samples consisted of one piece of epaxial white muscle taken from behind the head. Each sample was weighed, diluted 1:10 (mg:μl) in 0.1 M sodium phosphate buffer (pH 8.0) with 0.5% Triton X-100. Tissues were homogenized for 1 min using a glass douncer on ice. Homogenates were centrifuged at 4 °C for 10 min at 7000 × g to remove large particulate material. The supernatant fraction was transferred to a separate tube and the total protein concentration was determined with the Biorad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA) using methods of Lowry et al. (1951). For the AChE assay, 0.1 M sodium phosphate buffer (pH 8.0) with 0.5% Triton X-100 was added to the supernatant fractions to produce final dilutions of 1:500 (mg:μl) for muscle samples and 1:200 (mg:μl) for brain samples. Assay optimization was performed with brain and muscle tissue from unexposed juvenile Chinook salmon. Acetylthiocholine iodide (AtChI) concentrations between 0.1 and 5 mM were tested for optimal substrate concentration, and samples were incubated with tetraisopropylpyrophosphoramide (iso-OMPA, a selective AChE inhibitor) to measure butyrylcholinesterase-mediated substrate hydrolysis. Results showed negligible butyrylcholinesterase activity in muscle tissue, therefore subsequent assays were performed without the AChE inhibitor.
AChE activity in brain and muscle was analyzed using modified methods of Ellman et al. (1961). AChE activity for each sample was determined by adding 30 μl of diluted supernatant to a microplate well (Costar 96 well EIA/RIA Plate; Corning Inc., New York, NY) containing 250 μl of 0.1 M sodium phosphate buffer (pH 8.0), 10 μl of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB, 10.3 mM), and 30 μl of AtChI (21.4 mM). Final assay concentrations were 0.32 mM DTNB and 2 mM AtChI. Final protein concentrations ranged from 10.8 to 17.1 μg/μl for muscle and 7.0 to 10.7 μg/μl for brain. All assays were performed in triplicate. Absorbance at 412 nm was measured at 2 min intervals for 10 min at 25 °C with an automated microplate reader (Model EL3401; Bio-Tek Instruments, Winooski, VT) and all samples were corrected for background hydrolysis. AChE activity was calculated as μmol/min/g wet weight, and then normalized to the amount of protein in the homogenate (μmol/min/mg protein).
2.5. Carboxylesterase assays
Livers were excised and processed as described above using Tris buffer (pH 8.0, 20 mM) containing 5 mM EDTA. Samples were centrifuged at 9000 g for 20 min at 4 °C. Esterase assays with p-nitrophenylacetate (PNPA) were performed using sodium phosphate buffer (pH 8.0, 0.1 M) at 30 °C according to methods of Wheelock et al. (2001) as adapted fromLjungquist and Augustinsson (1971). Assays were pre-formed in 96-well microtiter styrene flat bottom plates (Dynex Technologies, Chantilly, VA) and analyzed on a Spectramax 340PC plate reader (Molecular Devices, Sunnyvale, CA). The total assay volume was 200 μl, consisting of 180 μl buffer and 20 μl of enzyme preparation. All assays were designed such that no more than 10% of the substrate was hydrolyzed over the length of the assay and solvent content never exceeded 1% of the total assay volume. Reported results are all corrected for background hydrolysis of the substrate. Activity was monitored using a 2.0 min kinetic read at 405 nm. The amount of protein added in each assay varied by species and sample, but ranged from 29 to 158 μg/well for the salmon liver, 10 μg/well for splittail, 25 μg/well for medaka and 4.4 μg/well for porcine esterase. Protein concentration was adjusted such that the assay was linear over the reported time interval.
All assays with α-cyanoester substrates were performed as described in Wheelock et al. (2003). Fluorescent assays were conducted with a Spectrafluor Plus (Tecan, Research Triangle, NC) running Magellan v. 2.50 software. Assays were conducted in black 96-well polystyrene flat clear bottom microtiter plates (Corning Inc.) at 30 °C. The total assay volume was 200 μl, consisting of 180 μl Tris buffer (pH 8.0, 20 mM) and 20 μl of enzyme preparation. Substrate solutions were prepared in ethanol (10 mM) and assays were initiated by the addition of 2 μl substrate solution followed by shaking for 10 s. Production of 6-methoxynaphthaldehye was monitored with excitation at 330 nm (bp 35) and emission at 465 (bp 35). All assays were performed with the instrument gain set to 60. Assays were configured such that no more than 10% of the substrate was hydrolyzed during the assay and solvent added never exceeded 1% of the total assay volume. Reported activities were corrected for background hydrolysis. For each species examined, standard curves were generated by adding an equivalent amount of protein to each standard concentration to account for protein-induced aldehyde quenching. It is important that standard curves are generated in the presence of authentic protein samples on a species-specific basis. Assays were performed with three flashes and 10 cycles to give a ∼3 min linear assay. The amount of protein added in each assay varied with the species and sample, but ranged from 5.6 to 44 μg/well for salmon liver, 10 μg/well for splittail, 25 μg/well for medaka and 0.08 μg/well for porcine esterase. Protein concentration was adjusted such that the assay was linear over the reported time. A series of aliphatic and pyrethroid surrogate fluorescent substrates developed in our laboratory were screened for esterase-mediated hydrolysis with the different species examined in this study. A full description of these substrates, including their synthesis and use as pyrethroid surrogates, is described by Stok et al. (2004).
Kinetic constants were measured using the assays described above for each substrate. A range of solutions of varying substrate concentration were prepared in ethanol. Total solvent never exceeded 1% of the assay volume. Kinetic constants were calculated using a nonlinear curve fit with a minimum of nine substrate concentrations as described by Segel (1976).
Assays to determine the concentration of inhibitor that reduced enzyme velocity by 50% (IC50) were performed according to methods of Wheelock et al. (2004) and designed such that there were at least two datum points above and below the IC50 value in the linear range. Inhibitor solutions were prepared in ethanol and diluted as required for each assay. Solvent never exceeded 1% of the assay volume and no solvent effects were observed.
2.6. Native gel activity assay
Native polyacrylamide gel-electrophoresis (PAGE) analyses were performed using 12% tris-glycine gels (Invitrogen) according to the manufacturer’s instructions. Gels were visualized with the carboxylesterase activity stain α-naphthyl acetate using methods of Huang et al. (1993), and scanned with a UMAX Powerlook III flatbed scanner (UMAX Technologies Inc., Dallas, TX).
2.7. Immunoblotting procedures for determination of CYP1A levels
CYP1A protein was quantified by immunoblotting using a Bio-Dot SF™ microfiltration slot-blot apparatus (Bio Rad, Hercules, CA) according to published procedures (McArdle et al., 2004). Briefly, 20 μg of S9 protein was suspended in 200 μl of 1 TBS (pH 7.5, 20 mM Tris, 0.5 M NaCl), loaded into each well and vacuum transferred onto a nitrocellulose membrane (0.45 μM; Schleicher and Schull, Keene, NH). The membrane was incubated in 1 TBS-5% milk for 1 h at room temperature to block non-specific binding, followed by incubation with MAb 1-12-3 (1:50), a monoclonal antibody which recognizes CYP1A in multiple vertebrate species (Stegeman and Hahn, 1994). CYP1A signal was detected using Cy™ 5-conjugated affinipure goat anti-mouse IgG as the secondary antibody (Jackson Immunoresearch Laboratories Inc., West Grove, PA) and blots were scanned at 633 nm excitation/670 nm emission using a Typhoon 8600 scanner (Molecular Dynamics, Palo Alto, CA) and quantified using Scion Image (NIST, http://www.nist.gov/lispix/imlab/labs.html). Liver microsomes from trout treated with the CYP1A model inducer, β-naphthoflavone (50 mg/kg, i.p.) were used as positive controls and loaded in seven concentrations (0.1–7.0 μg/well) to evaluate the linearity of the CYP1A signal on each blot. All samples were run in at least triplicate.
2.8. Statistical analyses
The significance of the effects of pesticide treatment upon esterase assays was analyzed using the statistical package in Microsoft Excel (Redmond, WA). Students t-test were performed, with significance reported for P <0.05. Michaelis–Menton kinetic analyses were performed using two different methods, a double-reciprocal plot (Segel, 1976) and SigmaPlot (Systat Software Inc., Richmond, CA). CYP1A data were analyzed using one-way analysis of variance (ANOVA) and the SAS statistical package (SAS, 1985) and homogeneity of variances was determined by Levene’s test (Draper and Hunter, 1969). Separation of means was tested using Duncan’s multiple range test. All differences were considered significant at P ≥ 0.05.
3. Results
3.1. Acute toxicity
Aqueous chlorpyrifos concentrations in exposure containers showed ∼20–25% variability between measured and dosed concentrations (Table 1). These data agreed with previous results in similar exposure systems that showed a <20% variability between nominal and measured concentrations (unpublished results). Exposure of juvenile Chinook salmon to a range of chlorpyrifos concentrations elicited a dose-dependent acute response with 100% mortality observed at 81 μg/l, 20% mortality at 7.3 μg/l and 0% mortality at 1.2 μg/l. Fish exposure to a range of esfenvalerate concentrations resulted in 100% mortality at 1.0 μg/l (nominal) or 0% mortality at 0.01 and 0.1 μg/l(nominal). All subsequent enzyme assays were performed on fish that were exposed to sublethal pesticide doses.
Table 1.
Juvenile Chinook salmon mortality following pesticide exposure
| Pesticide | Concentration (g/l)a | Mortalityb(%) |
|---|---|---|
| Chlorpyrifos | 0 | 0 |
| 1.0 (1.2) | 0 | |
| 10 (7.3) | 20 | |
| 100 (81) | 100 | |
| Esfenvalerate | 0 | 0 |
| 0.01 | 0 | |
| 0.1 | 0 | |
| 1.0 | 100 |
Water concentrations are nominal unless specified. Values in parentheses were quantified using methods of Crepeau et al. (2000). Unpublished work showed that esfenvalerate concentrations varied from nominal concentrations by ~20%.
Juvenile Chinook salmon were exposed to the indicated concentration of pesticide for 96 h (n = 10).
3.2. Acetylcholinesterase activity
AChE activity varied with pesticide concentration and tissue type (Table 2). Solvent (methanol) exposure altered AChE activity in the brain (11% decrease, P <0.001), but not in muscle, relative to untreated controls. Significant suppression of AChE activity by chlorpyrifos treatment occurred only at the highest chlorpyrifos dose. Relative to solvent controls, exposure to low doses of chlorpyrifos (1.2 μg/l) did not suppress brain AChE, and even slightly elevated muscle AChE levels (112%, P <0.05). In contrast, high dose chlorpyrifos exposure (7.3 μg/l) reduced AChE activity by 85% (brain) and 92% (muscle) compared to solvent controls, and by 84% (brain) and 93% (muscle) relative to low dose fish (P <0.001).
Table 2.
Acetylcholinesterase activity in pesticide-exposed juvenile Chinook salmona
| Pesticide (tissue) | Concentration (μg/l)b | Average ± S.D.c | Ranged |
|---|---|---|---|
| Chlorpyrifos (brain) | Controle | 206 ± 12 | 2.1 |
| Solventf | 183 ± 18 | 2.0 | |
| 1.2 | 168 ± 23 | 1.7 | |
| 7.3 | 27 ± 6* | 2.1 | |
| Chlorpyrifos (muscle) | Control | 158 ± 34 | 1.2 |
| Solvent | 162 ± 29 | 1.4 | |
| 1.2 | 180 ± 32 | 1.7 | |
| 7.3 | 13 ± 4* | 2.0 | |
| Esfenvalerate (brain) | Control | 206 ± 12 | 2.1 |
| Solvent | 183 ± 18 | 2.0 | |
| 0.01 | 197 ± 21 | 2.2 | |
| 0.1 | 195 ± 8 | 1.4 | |
| Esfenvalerate (muscle) | Control | 158 ± 34 | 1.2 |
| Solvent | 162 ± 29 | 1.4 | |
| 0.01 | 151 ± 33 | 1.5 | |
| 0.1 | 145 ± 20 | 1.1 |
Juvenile Chinook salmon were exposed to the indicated concentration of pesticide for 96 h as described in Section 2. Only the salmon exposed to 7.3 μg/l chlorpyrifos had significantly inhibited acetylcholinesterase activity.
All reported water concentrations are nominal values. Chlorpyrifos concentrations were measured to be 1.2 and 7.3 μg/l (as opposed to 1.0 and 10.0 μg/l nominal). Esfenvalerate concentrations were observed to deviate by ∼20% from nominal concentrations (unpublished results).
Activity values are in units of nmol/min/mg and are the average ± the standard deviation (S.D.) for 10 fish (n = 10), except for the 7.3 μg/l chlorpyrifos exposure (n = 8). Assays were performed in triplicate and variability was less than 10%.
Range values are given as the fold difference in activity between the lowest and highest individuals.
Control fish were not exposed to either pesticides or vehicle and did not go through the experimental testing regimen.
Solvent fish were exposed to the highest concentration of vehicle (0.005% MeOH) and went through the full 96 h testing regimen.
Statistically different from the control, solvent-exposed, and the 1.2 μg/l chlorpyrifos-exposed salmon (P < 0.001).
Esfenvalerate exposure did not affect AChE activity in either tissue at the lowest dose, 0.01 μg/l. However, at 0.1 μg/l, brain AChE activity increased by 10% and muscle AChE activity decreased by ∼10%. These differences, though slight, were significantly different from solvent controls (0.05 > P > 0.01). Activities at the two esfenvalerate exposure concentrations were not statistically different from each other.
Inter-individual variability in AChE activity was generally less in brain (∼10%) than in muscle (20%). The ratio in activity between the individual with the highest level of activity to the individual with the lowest ranged from 1.1 for esfenvalerate at 0.1 μg/l in muscle to 2.2 at 0.01 μg/l esfenvalerate in brain. The majority of the exposures showed no significant effect upon the range of inter-individual activity, with the exception of activity in the muscle after chlorpyrifos exposure. This sample showed a steady increase in the range of activity with treatment. However, the overall change was still relatively small (from 1.2- to 2-fold).
3.3. Carboxylesterase assays
Pesticide exposure affected carboxylesterase activity in a compound and dose-dependent fashion (Table 3). All three substrates examined produced very similar results, with hydrolysis activity profiles for each substrate responding identically to pesticide exposure. Increasing concentrations of chlorpyrifos caused significant decreases in carboxylesterase activity (Table 3). An identical inhibition pattern was observed using an α-naphthyl acetate carboxylesterase activity stain in a native gel (Fig. 3). Significant reductions in carboxylesterase activity at the highest chlorpyrifos dose (7.3 μg/l) were detected by all three substrates, with reductions of 79% (PNPA), 52% (α-cyano acetate) and 55% (α-cyano butyrate), relative to solvent controls. Only one substrate, PNPA, detected a significant reduction (56%) in carboxylesterase activity at the low chlorpyrifos dose (1.2 μg/l; P <0.001). Solvent exposure had a significant effect upon carboxylesterase activity for all three substrates, with reductions ranging from a 44% decrease in activity for the α-cyanoacetate to 23% for PNPA, relative to untreated controls. The α-cyano acetate and α-cyano butyrate substrates exhibited a wider range of inter-individual carboxylesterase activities (2.4–3.0- and 1.3–3.9-fold, respectively), than did PNPA (1.3–1.8-fold).
Table 3.
Carboxylesterase activity in liver cytosol from pesticide-exposed juvenile Chinook salmona
| Pesticide | Substrate | Concentration (μg/l)b | Average ± S.D.c | Ranged |
|---|---|---|---|---|
| Controle | PNPAf | 0 | 85.9 ± 22.0 | 2.2 |
| Acetateg | 0 | 20.8 ± 7.6 | 2.4 | |
| Butyrateh | 0 | 4.5 ± 1.0 | 1.9 | |
| Chlorpyrifos | PNPA | Solventi | 66.4 ± 11.0** | 1.7 |
| 1.2 | 29.4 ± 6.3* | 1.8 | ||
| 7.3 | 13.7 ± 1.2* | 1.3 | ||
| Acetate | Solvent | 11.6 ± 3.3** | 3.0 | |
| 1.2 | 13.4 ± 4.3 | 2.9 | ||
| 7.3 | 5.6 ± 1.7* | 2.4 | ||
| Butyrate | Solvent | 3.1 ± 0.3** | 1.4 | |
| 1.2 | 2.6 ± 0.9 | 3.9 | ||
| 7.3 | 1.4 ± 0.1* | 1.3 | ||
| Esfenvalerate | PNPA | Solvent | 73.1 ± 26.2 | 2.9 |
| 0.01 | 56.8 ± 11.4 | 1.8 | ||
| 0.1 | 63.7 ± 10.7 | 1.6 | ||
| Acetate | Solvent | 17.6 ± 8.0 | 3.3 | |
| 0.01 | 11.4 ± 3.9† | 3.0 | ||
| 0.1 | 13.5 ± 2.6 | 1.8 | ||
| Butyrate | Solvent | 4.0 ± 1.3 | 2.3 | |
| 0.01 | 3.0 ± 0.9 | 3.1 | ||
| 0.1 | 3.2 ± 0.4 | 1.5 |
Juvenile Chinook salmon were exposed to the indicated concentration of pesticide for 96 h as described in Section 2.
Esfenvalerate concentrations are nominal values and chlorpyrifos concentrations are measured.
Activity values are in units of nmol/min/mg and are the average ± the standard deviation (S.D.) for 10 fish (n = 10), except for the 7.3 g/l chlorpyrifos exposure (n = 8).
Range values are given as the fold difference in activity between the lowest and highest individuals.
Control fish were not exposed to either pesticides or vehicle and did not go through the experimental testing regimen.
Carboxylesterase activity assays were performed with the substrate p-nitrophenyl acetate (PNPA).
Carboxylesterase activity assays were performed with the substrate -cyano(6-methoxy-2-naphthyl)methyl acetate (acetate).
Carboxylesterase activity assays were performed with the substrate -cyano(6-methoxy-2-naphthyl)methyl butyrate (butyrate).
Solvent fish were exposed to the highest concentration of vehicle (0.005% MeOH) and went through the full 96 h testing regimen.
Statistically different from the solvent-exposed salmon (P < 0.001).
The solvent-exposed (MeOH vehicle) salmon were statistically different from the controls (P < 0.01).
The value is statistically different from the solvent-exposed salmon (P < 0.05).
Fig. 3.
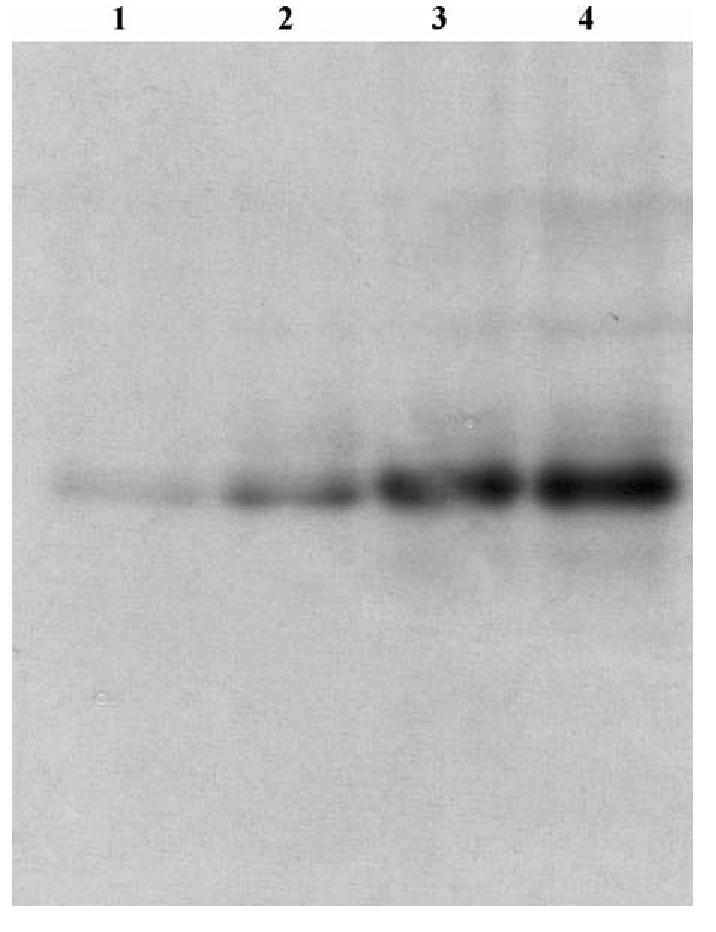
Carboxylesterase activity in liver cytosol from chlorpyrifos-treated juvenile Chinook salmon. Activity was visualized in a 12% tris-glycine native gel stained with α-naphthyl acetate. Lane 1: chlorpyrifos-exposed at 10 μg/l; lane 2: chlorpyrifos-exposed at 1.0 μg/l; lane 3: chlorpyrifos solvent control; lane 4: control unexposed fish.
Exposure to esfenvalerate had very little effect upon carboxylesterase activity. All concentrations tested were essentially identical to solvent control values for all substrates, and no significant solvent effects were observed (Table 3). Exposure to 0.01 and 0.1 μg/l esfenvalerate inhibited carboxylesterase activities relative to untreated controls (P <0.05) when measured with the α-cyano acetate substrate, however values were not significantly different from the solvent control. The range in inter-individual variation in carboxylesterase activity in response to esfenvalerate exposure was similar to that observed with chlorpyrifos. Generally, the range in activity decreased with increasing pesticide concentration with all three substrates. The only exception occurred with the butyrate substrate in which the lowest concentrations of chlorpyrifos and esfenvalerate elicited the greatest range of activity.
3.4. Determination of carboxylesterase kinetic constants in different species
The carboxylesterase kinetic constants varied with species and substrate. It is not appropriate to directly compare species in this study as the tissues were prepared differently and thus limited conclusions can be drawn from the data. For medaka and splittail, whole body homogenates were used, for salmon, liver homogenates were used, and for porcine carboxylesterase, measurements were made using a partially purified commercial esterase. Because purified carboxylesterases were not used for any species, kinetic constants are reported as apparent values. The Michaelis constant (Km app) for PNPA varied from a low of 95 μM for medaka to a high of 578 μM for split-tail (a six-fold range) as shown in Table 4. Results for the α-cyanoacetate and butyrate substrates were more similar to each other than to PNPA. Km app values were ∼30 μM for the acetate for all species except split-tail (5.8 μM), whereas butyrate values ranged from 7.8 to 16.8 μM. Interestingly, values for the salmon and porcine enzymes fell within the range observed for medaka, splittail and trout, even though the salmon and porcine enzymes were prepared from single organs (salmon) or were partially purified (porcine) and had higher specific activities.
Table 4.
Kinetic constants for substrates used in this studya
| Organism | Km app (μM) | Vmax app (nmol/min/mg) |
|---|---|---|
| p-Nitrophenyl acetateb | ||
| Salmon | 154 ± 13 | 363,000 ± 14,000 |
| Medaka | 95 ± 12 | 198,000 ± 19,000 |
| Splittail | 578 ± 30 | 129,600 ± 4300 |
| Rainbow troutc | 27.9 ± 12.7 | 672,000 ± 92,100 |
| Porcine esterase | 248 ± 17 | 2,103,000 ± 67,000 |
| Acetated | ||
| Salmon | 30.4 ± 6.1 | 270 ± 23 |
| Medaka | 30.3 ± 8.2 | 29.2 ± 3.4 |
| Splittail | 5.8 ± 1.1 | 7.0 ± 0.4 |
| Porcine esterase | 29.2 ± 2.3 | 2800 ± 100 |
| Butyratee | ||
| Salmon | 7.8 ± 1.0 | 77.4 ± 2.8 |
| Medaka | 16.8 ± 2.0 | 8.7 ± 0.3 |
| Splittail | 8.2 ± 1.7 | 16.4 ± 1.0 |
| Porcine esterase | 12.2 ± 2.5 | 16,100 ± 1100 |
Salmon data are from liver homogenates. Medaka and splittail data are from whole body homogenates, and porcine esterase data are from a commercial partly purified preparation. Data are the average of 3 independent determinations ± the standard deviation.
Kinetic constants were determined for the substrate p-nitrophenyl acetate (PNPA).
Data are from Barron et al. (1999) using rainbow trout liver.
Kinetic constants were determined for the substrate α-cyano(6-methoxy-2-naphthyl)methyl acetate (acetate).
Kinetic constants were determined for the substrate α-cyano(6-methoxy-2-naphthyl)methyl butyrate (butyrate).
In contrast to the Michaelis constant, the variation in Vmax app was much greater among the different species examined. This variability tracked carboxylesterase concentration in the preparation very closely, as Vmax app values are normalized to unit protein. The two samples that were prepared identically (whole body homogenates) had similar Vmax app values for PNPA, 198,000 nmol/min/mg for medaka and 129,600 nmol/min/mg for splittail, whereas the samples that contained a higher degree of specific activity (salmon liver or porcine esterase) had Vmax app values that were up to 10-fold higher. Similar results were reported for studies with rainbow trout liver preparations, which reported a Vmax app for PNPA of 672,000 nmol/min/mg (Barron et al., 1999). Similar trends were observed with both the α-cyanoacetate and butyrate substrates.
3.5. Measurement of inhibitor potency
The OP pesticides diazinon and chlorpyrifos, and their oxon-derivatives, were examined for their ability to inhibit carboxylesterase activity in four species using three different substrates (Table 5). The IC50’s (concentration of enzyme required to reduce enzyme velocity by 50%) for diazinon and chlorpyrifos were greater than 100 μM for all three substrates in all four species, indicating that these two pesticides do not significantly inhibit carboxylesterase activity. In contrast, the oxon forms of both pesticides were significant carboxylesterase inhibitors, with IC50 values in the low nM range for all substrates in all species examined, except splittail. In splittails, diazinon-oxon did not inhibit α-cyano acetate hydrolysis at any concentration examined (IC50 > 100 μM) while chlorpyrifosoxon mediated inhibition was as much as 1000-fold less potent than in the other species tested.
Table 5.
Inhibition concentrations (IC50) for selected organophosphatesa
| Organism | Diazinon | Diazinon-oxon | Chlorpyrifos | Chlorpyrifos-oxon |
|---|---|---|---|---|
| p-Nitrophenyl acetateb | ||||
| Salmon | >100c | 3.54 ± 0.15 | >100 | 0.14 ± 0.02 |
| Medaka | >100 | 39.5 ± 2.4 | >100 | 6.12 ± 0.30 |
| Splittail | >100 | 20.1 ± 1.4 | >100 | 8.42 ± 0.38 |
| Porcine esterase | >100 | 1.35 ± 0.08 | >100 | 1.55 ± 0.05 |
| Acetated | ||||
| Salmon | >100 | 8.56 ± 0.97 | >100 | 0.55 ± 0.04 |
| Medaka | >100 | 28.8 ± 2.5 | >100 | 5.25 ± 0.18 |
| Splittail | >100 | > 100 | >100 | 18.9 ± 0.9e |
| Porcine esterase | >100 | 1.43 ± 0.17 | >100 | 0.74 ± 0.08 |
| Butyratef | ||||
| Salmon | >100 | 33.5 ± 0.35 | >100 | 2.39 ± 0.11 |
| Medaka | >100 | 51.8 ± 3.3 | >100 | 5.53 ± 0.28 |
| Splittail | >100 | 21.8 ± 2.6 | >100 | 2.55 ± 0.21 |
| Porcine esterase | >100 | 1.71 ± 0.15 | >100 | 1.01 ± 0.113 |
All IC50 concentrations are in nM unless otherwise noted. Salmon data are from liver homogenates. Medaka and splittail data are from whole body homogenates, and porcine esterase data are from a commercial partly purified preparation. Data are the average of 3 independent determinations ± the standard deviation.
IC50 determinations were performed with the substrate p-nitrophenyl acetate (PNPA).
All values for >100 are in μM.
IC50 determinations were performed with the substrate α-cyano(6-methoxy-2-naphthyl)methyl acetate (acetate).
IC50 values are in μM.
IC50 determinations were performed with the substrate α-cyano(6-methoxy-2-naphthyl)methyl butyrate (butyrate).
Of the two oxons tested, chlorpyrifos-oxon was the more potent carboxylesterase inhibitor, being on average ∼10-fold more potent than diazinon-oxon. However, this number varied considerably with substrate and species. For medaka and the porcine esterase, the IC50s for the two different oxons did not vary greatly with substrate. However, for the Chinook salmon esterase, the two acetate-containing substrates, PNPA and α-cyano acetate, had IC50s more similar to one another than to the IC50 for the α-cyano butyrate substrate. This observation is in spite of the fact that the alcohol moiety of α-cyano acetate is quite different from that of PNPA, being similar to α-cyano butyrate (see Figs. 1 and 2 for a description of acid and alcohol nomenclature and substrate structures).
3.6. Pyrethroid hydrolysis
Pyrethroid surrogate hydrolysis was not observed with any of the fish species examined in this study. Only the porcine enzyme significantly hydrolyzed α-cyanoesters of pyrethroid acids as shown in Table 6. A number of assay conditions were varied in an attempt to measure hydrolysis activity. The temperature of the assay was increased up to 37 °C and the pH up to 9; however none of these conditions was sufficient to increase pyrethroid hydrolysis activity to quantifiable levels. Unless otherwise stated, the substrates were a mixture of isomers. The hydrolysis rates of the eight different pyrethroid surrogates examined did not vary by more than ∼10-fold across all compounds (3.78-38.16 nmol/min/mg). For compound 1, an esfenvalerate mimic, hydrolysis was ∼2.5-fold slower than for the corresponding R-analog (compound 2). The cypermethrin surrogate (compound 3) was hydrolyzed at the same rate as its dimethyl analog (synthesized from chrysanthemic acid, compound 7); however substitution of the dichloro moiety by dibromo (deltamethrin surrogate) decreased hydrolysis by 7-fold (com-pound 4). The λ-cyhalothrin surrogates∼exhibited a 4-fold difference in hydrolysis rate, with the trans isomer (compound 5) hydrolyzed faster than the cis (com-pound 6).
Table 6.
Hydrolysis activity of synthetic pyrethroid surrogate substrates
| Compounda | Substrate | Esterase activityb |
|---|---|---|
| 1 | 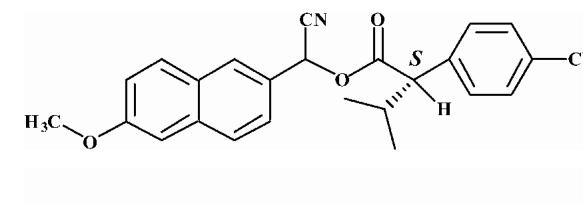 |
8.46 ± 0.09 |
| 2 | 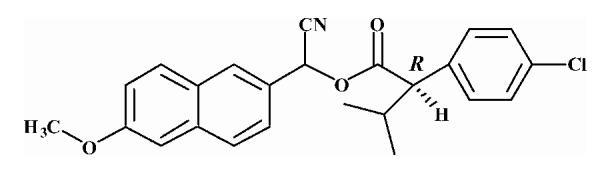 |
20.78 ± 0.68 |
| 3 | 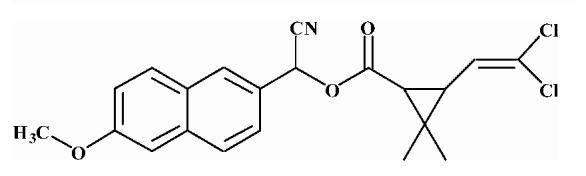 |
37.72 ± 0.70 |
| 4 | 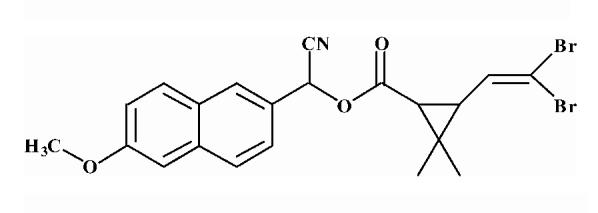 |
5.03 ± 0.26 |
| 5 | 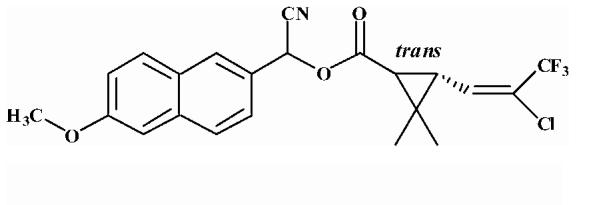 |
14.69 ± 0.43 |
| 6 | 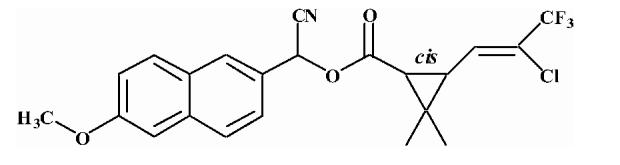 |
3.78 ± 0.10 |
| 7 | 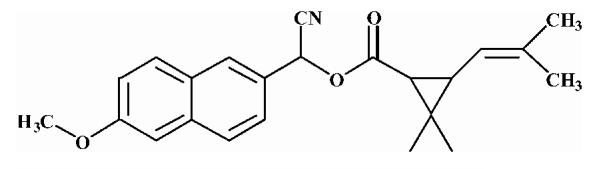 |
38.16 ± 0.84 |
| 8 | 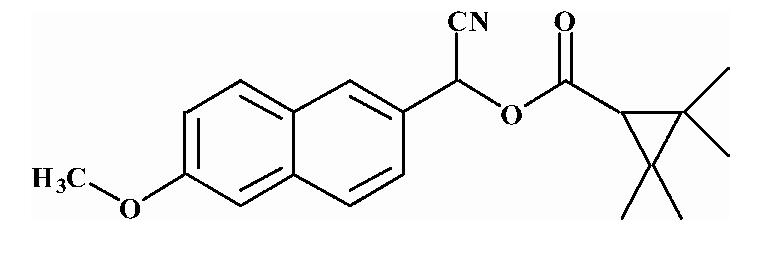 |
6.50 ± 0.28 |
Substrates are pyrethroid surrogates: compound 1 for esfenvalerate, 2 for fenvalerate, 3 for cypermethrin, 4 for deltamethrin, 5 for trans λ-cyhalothrin, 6 for cis λ-cyhalothrin, 7 for phenothrin, and 8 for fenpropathrin.
Activity is in nmol/min/mg protein using a commercial porcine esterase. No significant hydrolysis activity of the substrates (1-8) could be detected with any of the fish tissues examined in this study. Data are the average of 3 independent determinations ± the standard deviation.
3.7. CYP1A levels
CYP1A protein expression was slightly, but significantly (P <0.05), suppressed (1.4-fold) in salmon liver cytosol from the high dose (7.3 μg/l) chlorpyrifos-treated group relative to those treated with solvent carrier (Table 7). However, there was no significant effect of chlorpyrifos at either dose relative to untreated controls. Esfenvalerate treatment had no effect on hepatic CYP1A protein expression at either dose (Table 7). No significant trends were observed in the range of CYP1A levels in response to the different pesticide exposures. The range varied from a low of 1.6-fold for the highest dose of esfenvalerate (similar to the control value of 1.7) to a high of 4.3 for the highest dose of chlorpyrifos. However, variability in response to low dose esfenvalerate (4.2) was similar to that provoked by the high dose of chlorpyrifos (4.3), suggesting salmon response to these pesticides was generally similar. The variability in response to solvent exposure was moderate, with a range of 2.5 and 2.6 for esfenvalerate- and chlorpyrifos-exposed fish, respectively, ranges higher than the response range for untreated controls (1.7).
Table 7.
Relative levels of CYP1A protein in liver cytosol from pesticide-exposed Chinook salmona
| Pesticide | Concentration (μg/l)b | Average ± S.D.c | Ranged |
|---|---|---|---|
| Controle | 0 | 1710 ± 310 | 1.7 |
| Chlorpyrifos | Solventf | 2060 ± 440 | 2.6 |
| 1.2 | 1960 ± 360 | 1.9 | |
| 7.3 | 1480 ± 560* | 4.3 | |
| Esfenvalerate | Solvent | 1600 ± 430 | 2.5 |
| 0.01 | 1390 ± 500 | 4.2 | |
| 0.10 | 1460 ± 420 | 1.6 |
Juvenile Chinook salmon were exposed to the indicated concentration of pesticide for 96 h as described in Section 2. All treatment groups were statistically similar to untreated controls (P < 0.05).
Esfenvalerate concentrations are nominal values and chlorpyrifos concentrations are measured.
Relative CYP1A protein levels are the average ± the standard deviation (S.D.) for 10 fish (n = 10), except for the 7.3 μg/l chlor-pyrifos exposure (n = 8).
Range values are given as the fold difference in relative protein levels between the lowest and highest individuals.
Control fish were not exposed to either pesticides or vehicle and did not go through the experimental testing regimen.
Solvent fish were exposed to the highest concentration of vehicle (0.005% MeOH) and went through the full 96 h testing regimen.
Statistically different from the solvent-exposed salmon (P < 0.05).
4. Discussion
Early studies suggested that fish metabolize xenobiotics at much slower rates than mammals, if at all. More recently it has become clear that xenobiotic metabolism in fish species is often different from that in mammals, but often very active nonetheless. Extensive work has examined cytochrome P450 (Stegeman and Lech, 1991; Goksoyr, 1995; Whyte et al., 2000), AChE (McKim et al., 1987; Fulton and Key, 2001), and glutathione transferase (GST) (Parker et al., 1993; Bello et al., 2001) activity in a range of fish species. However, to date relatively little information is available on carboxylesterase activity, even though these enzymes interact with many agrochemicals including pyrethroids, OPs, and carbamates.
Carboxylesterases have been intensively studied in mammalian systems due to their role in mediating agrochemical-induced toxicity (Satoh and Hosokawa, 1998; Sogorb and Vilanova, 2002; Stok et al., 2004; Wheelock et al., 2005). Research has shown that carboxylesterases reduce pyrethroid-associated toxicity (Abernathy and Casida, 1973) and that joint exposure to pyrethroids and/or carbamates can cause synergistic toxicity (Gaughan et al., 1980; Gupta and Dettbarn, 1993). Early work on fish by Kingsbury and Masters (1972) reported the presence of three carboxylesterase isozymes in rainbow trout with the detection of one polymorphism. Since then a number of researchers have examined esterase activities in fish, but the work lags significantly behind that performed in mammalian systems (Boone and Chambers, 1997; Huang et al., 1997; Sanchez-Hernandez et al., 1998; Barron et al., 1999; Al-Ghais, 2000; Wogram et al., 2001; Denton et al., 2003). This study expands the current literature on carboxylesterases in several fish species and examines the appropriateness of multiple biochemical endpoints as biomarkers of agrochemical exposure and/or susceptibility.
4.1. Acute toxicity
Significant fish mortality at the highest concentrations of both pesticide exposures was anticipated and is consistent with results from toxicity studies in related fish species. Since 96-h LC50 values for chlorpyrifos and esfenvalerate in juvenile Chinook salmon have not been reported in the literature, we chose concentrations that bracketed those used in studies with rainbow trout, a related species. The 96-h LC50 for chlorpyrifos has been reported to range from 9 μg/l (Phipps and Holcombe, 1985) to 45 μg/l (Kikuchi et al., 1996) in juvenile rainbow trout. For esfenvalerate, only two 96-h LC50 studies have been published for rainbow trout; 0.3 μg/l (Du Pont, 2002) and 0.07 μg/l (U.S. EPA, 2000). Based on these limited data, we chose a wide range of pesticide concentrations for our studies to identify doses that were acutely toxic and doses that would potentially elicit sublethal toxicity. Our results for chlorpyrifos- and esfenvalerate-induced acute mortality identified concentrations within the general range of 96-h LC50 concentrations reported for rainbow trout, suggesting Chinook salmon and rainbow trout have similar sensitivities to these pesticides.
4.2. Acetylcholinesterase activity
Inhibition of AChE activity is linked directly with the mechanism of toxic action of OP insecticides, and thus is often used as an indicator of OP exposure and physiological effect in exposed animals (Fulton and Key, 2001). The AChE activity data (Table 2) from this study suggest either that multiple isoforms of the enzyme do not exist, or, if they do, that all have similar sensitivity to chlorpyrifos. We found good agreement between the average AChE activity in brain of the control group and mean control activity in a study with juvenile steelhead trout, with 14.9 μmol/min/g brain tissue in salmon and 15.3 μmol/min/g brain tissue in steelhead (expressed as wet weight) (Sandahl and Jenkins, 2002).
Inhibition of AChE activity in fish is generally correlated with increased mortality; however differences among species and between tissues make it difficult to identify the lowest level of AChE inhibition that can cause mortality. The sensitivity of brain and muscle AChE, the relationship between tissue-specific AChE inhibition and mortality, as well as the presence and enzymatic activity of butyrylcholinesterase, all appear to be species-specific (Fulton and Key, 2001). For example, in most studies with estuarine fish, inhibition levels in excess of 70% correlate with imminent mortality, but inhibition of AChE activity greater than 80% in surviving fish is not uncommon (Coppage and Matthews, 1975; Coppage et al., 1975). This observation might be due to species-specific differences in tolerance to extremely high levels of brain AChE inhibition (Keizer et al., 1995). For this reason, it has been suggested that muscle AChE inhibition, which exhibits less species-specific differences than brain AChE inhibition, might be a more appropriate predictor of OP-related mortality (Fulton and Key, 2001). In our study, inhibition of brain and muscle AChE following exposure to 7.3 μg/l chlorpyrifos was roughly equal. However, at the lower chlorpyrifos dose (1.2 μg/l) AChE inhibition was only observed in the brain, suggesting that this tissue is a more sensitive indicator of OP insecticide exposure than muscle at concentrations well below those causing mortality. Results from similar studies (Fulton and Key, 2001) suggest that mortality is likely to occur when brain AChE inhibition reaches 70–80%. However, we observed little mortality (20%) at chlorpyrifos doses (7.3 μg/l) that substantially inhibited AChE activity (85% and 92% inhibition in brain and muscle, respectively).
OP insecticide concentrations in California rivers continue to exceed water quality standards (Werner et al., 2000; Phillips et al., 2004), and chlorpyrifos concentrations as high as 3.2 μg/l have been reported for the Central California Coast (Hunt et al., 2003). However, measured concentrations are generally below levels that cause mortality in laboratory studies, and it is uncertain to what extent environmental chlorpyrifos concentrations affect AChE activity. A number of studies reported a link between AChE inhibition in fish and sublethal behavioral and physiological effects, such as reduced swimming stamina (Post and Leasure, 1974; Van Dolah et al., 1997), altered feeding (Wildish and Lister, 1973; Bull and McInerney, 1974), and altered social interactions (Symons, 1973). In mammalian and avian systems, it has been suggested that AChE may play a direct role in the development of the nervous system (Brimijoin and Koenigsberger, 1999; Lauder and Schambra, 1999). It is therefore possible that inhibition of AChE activity at sublethal levels is having an adverse effect upon fish health and ecological viability.
4.3. Carboxylesterase activity
Carboxylesterase activity exhibited a dose– response relationship, with activity decreasing with increasing chlorpyrifos concentration (Tables 2 and 3). However following exposure to 7.3 μg/l chlorpyrifos, a significant amount of carboxylesterase activity (∼65%) was still observed with the α-cyano acetate and butyrate substrates (as opposed to PNPA, which had 21% remaining). This observation suggests the presence of multiple isozymes, with a significant level of activity (40–50%) not sensitive to chlorpyrifos inhibition. These data agree with results reported by Denton et al. (2003) who showed that exposure to diazinon resulted in a maximum of ∼50% inhibition of carboxylesterase activity in fathead minnows in vivo. Even though esfenvalerate did not cause any significant inhibition of carboxylesterase activity in our studies, the range in activities amongst the 10 fish did significantly drop by as much as 50%. This observation suggests that esfenvalerate has a previously unknown effect upon carboxylesterase isozyme abundance. These data also suggest that one set of pesticide sensitive isozymes has a wide range of variability, but that a second set of pesticide insensitive isozymes has a narrower range of variability. This variability in activity could be important in determining the effects of OP and/or carbamate exposure, because some individuals may be more sensitive to pesticide exposure due to lower levels of detoxifying carboxylesterases or possible polymorphisms.
The data in Table 3 show the effect of measuring carboxylesterase activity with different substrates and demonstrate that it is not appropriate to use a single substrate to examine activity of crude tissue homogenate. Given that there are likely multiple esterase isoforms present in the preparation, it is necessary to have a range of reporters of activity for full characterization. Generally the substrate PNPA, or other analogs using p-nitrophenol as a reporter, is employed due to its ease of use, availability and colored hydrolysis product. However, it is likely that there are additional esterase isozymes that do not hydrolyze this substrate. Correlation analyses performed on PNPA hydrolysis and pyrethroid hydrolysis in human liver showed very little correlation between the hydrolytic profiles (r2 = 0.29 for a fenvalerate surrogate) (Wheelock et al., 2003). These results suggest that different enzymes are involved in the hydrolysis of the different substrates. Therefore monitoring of PNPA activity, or that of other general substrates, may not provide an accurate account of pyrethroid hydrolysis activity. In this study, PNPA appeared to be a more sensitive indicator of activity/inhibition than the α-cyano substrates, reporting significant inhibition at the lowest level of chlorpyrifos examined. However, the α-cyano acetate and butyrate substrates exhibited a wider range in activities than PNPA, suggesting that they are hydrolyzed by more isozymes than PNPA.
All of the fish species examined in this study were unable to hydrolyze the pyrethroid surrogate substrates shown in Table 6 at significant levels. Substrates such as those developed by Riddles et al. (1983), which couple the leaving group p-nitrophenol to pyrethroid acids may be improved general reporters of pyrethroid hydrolysis activity compared to PNPA. They could be useful tools for determining if specific environmental contaminants interfered with an organism’s ability to hydrolyze pyrethroids, versus an overall measurement of general esterase activity. However, these surrogates still vary greatly in the alcohol portion from commercial pyrethroids and the substrates reported by Stok et al. (2004) are probably more appropriate pyrethroid surrogates. Ultimately, the actual pesticide should be used to test for activity.
The importance of substrate choice for monitoring esterase activity was further demonstrated by the IC50 data in Table 5. Inhibition assays with the split-tail homogenate showed a very striking result in that diazinon-oxon was a potent inhibitor (nM IC50 values) when assays were run with either PNPA or α-cyano butyrate. However, when assays were run with α-cyano acetate, no inhibition was observed. Similar results were observed with the chlorpyrifos-oxon assays. These data strongly suggest that a battery of substrates should be employed when measuring carboxylesterase activity to ensure that an accurate indication of enzyme activity is obtained.
It is possible that the ability to detoxify pyrethroids via hydrolysis is inversely correlated with pyrethroid toxicity, but there are not currently enough data available in the literature to fully examine this issue. Carboxylesterase activity is most likely important for pyrethroid detoxification in some species of fish, but work by Glickman and coworkers showed that the most important factor in rainbow trout sensitivity to permethrin was target site sensitivity at the sodium channel (Glickman et al., 1981; Glickman and Lech, 1981, 1982). Rainbow trout had lower esterase activity than rats, and thus a decreased ability to hydrolyze permethrin. However, after inhibiting all measurable esterase activity in both rainbow trout and rat using an esterase inhibitor, the rainbow trout were still more sensitive to permethrin toxicity. It is still possible that in some cases, different species will have greater levels of esterase activity, which could affect the ability to detoxify agrochemicals. Glickman et al. (1979) showed that carp had higher levels of esterase activity and a greater ability to hydrolyze permethrin than rainbow trout, which could potentially account for observed inter-species differences in pyrethroid toxicity.
Carboxylesterase activity may be a more sensitive marker for agrochemical exposure than AChE activity. Many different groups have studied the use of AChE as a biomarker of exposure to agrochemicals, mostly OPs, but some work has focused on carbamates as well (Sturm et al., 2000; Fulton and Key, 2001; Galloway et al., 2002; Rickwood and Galloway, 2004; Bonacci et al., 2004). It is inconclusive if AChE activity alone is an appropriate biomarker of OP exposure (Rickwood and Galloway, 2004). A number of researchers have shown that many OPs have increased affinity for carboxylesterase over AChE, indicating that carboxylesterase would be preferentially inhibited over AChE following exposure to OPs and potentially carbamates (Gupta and Dettbarn, 1993; Escartin and Porte, 1997; Wogram et al., 2001; O’Neill et al., 2004). In other words, carboxylesterases act as a sink for OPs, thereby rescuing AChE from OP toxicity. Work by Escartin and Porte (1997) examined the use of both cholinesterase and carboxylesterase activity in the mussel Mytilus galloprovincialis to monitor pollution in an agricultural region of Spain with known applications of OPs and carbamates. They found that carboxylesterase activity was more sensitive to pesticide exposure and that seasonal variation in carboxylesterase activity correlated with pesticide load in the organism. Based upon their observations, they postulated that carboxylesterase activity could serve as a protective mechanism for OP inhibition of AChE. A similar result was reported by Wogram et al. (2001) using the three-spined stickleback (Gasterosteus aculeatus), who found that carboxylesterase was 13–17-fold more sensitive to paraoxon than AChE.O’Neill et al. (2004) stated that both cholinesterase and carboxylesterase activity declined in response to exposure to sewage effluent discharge in northwest England; however carboxylesterase was more severely inhibited. These studies support the concept of using carboxylesterase activity as a biomarker of OP/carbamate exposure either alone or in combination with AChE activity.
We had originally hoped to examine correlations between carboxylesterase activity and pyrethroid and OP toxicity. However, there is not enough information available in the literature to draw direct correlations between toxicity (such as LC50) and esterase activity. One could envision a linear free energy relationship that correlated carboxylesterase and/or AChE activity to a toxicity endpoint for a given pesticide or class of pesticides. It would be useful if studies examined esterase activity (both AChE and carboxylesterase) in organisms for which toxicity assays were being conducted in the hopes of eventually collecting sufficient data for the correlation analyses. It is likely that no single biological measurement or biomarker will serve as a universal reporter of exposure to agrochemicals. A logical approach would be the integration of multiple biological or physiological endpoints. Galloway et al. (2004) developed a multibiomarker approach that incorporates a suite of ecologically relevant biomarkers. They found that carboxylesterase activity was one of the most discriminating markers among polluted sites, with correlation coefficients as high as 0.93. However, one problem with this multibiomarker approach is that the analysis is labor intensive and expensive. A key advantage of a single end-point such as AChE or carboxylesterase activity is the ease of measurement and low cost. It may be necessary to have multiple layers of testing that depend upon the available funds and labor as well as the rigor of the answer required. However, with the increasing availability of assays designed for 96- or 384-well applications and the advent of robotics, one could design experiments to look at multiple enzymes in several species under different exposure conditions in a cost and time effective manner.
4.4. CYP1A levels
Induction of CYP1A is extensively used as an indicator of exposure and response to organic pollutants in teleost fish and other vertebrates (Stegeman and Hahn, 1994). However, suppression of CYP1A can complicate and limit its utility as a biomarker. For example, metals (Fent and Bucheli, 1994) and hormones (Elskus et al., 1992; Elskus, 2004) have been shown to suppress CYP1A expression in fish, making it imperative that reproductive stage, gender and the presence of metals be taken into consideration when interpreting CYP1A data. It is possible that pesticides can co-occur with CYP1A-inducing chemicals, making it critical that regulation of CYP1A by pesticides is understood.
As expected, CYP1A protein was not induced in juvenile Chinook salmon exposed to either chlorpyrifos or esfenvalerate. Aromaticity and planarity are generally considered two structural features important for chemical induction of CYP1A (Safe, 1990). Inducers with these features include planar chlorinated aromatic hydrocarbons, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and certain polychlorinated biphenyls, making it one of the most widely used biomarkers of these pollutants in vertebrates (Buchneli and Fent, 1995), including salmonids (Whyte et al., 2000). While both chlorpyrifos and esfenvalerate have chlorinated aromatic rings (Fig. 1), neither chemical exhibits planarity and would therefore not be expected to induce CYP1A.
Although CYP1A expression was not induced in the pesticide exposed fish in this study, chlorpyrifos and esfenvalerate did exhibit differential effects on salmon CYP1A. We found esfenvalerate had no effect on CYP1A protein levels, in keeping with others who report esfenvalerate had no effect on CYP1A mRNA levels in rat hepatocytes (Heder et al., 2001), or on CYP1A catalytic activity (ethoxyresorufin-O-deethylase, EROD) levels in adult rainbow fish (Barry et al., 1995). There is some evidence that esfenvalerate affects CYP forms in fish other than CYP1A (Barry et al., 1995), suggesting its effects may be isozyme-specific.
Chlorpyrifos had little effect on CYP1A protein levels in salmon, suggesting OPs may not be strong regulators of CYP1A in fish. We know of no other studies examining chlorpyrifos effects on CYP1A protein in vertebrates. However, studies with the OP methidathion found that it slightly increased CYP1A protein levels in cyprinids after 4 days of aqueous exposure (Flammarion et al., 1998). This observation is in contrast to our present study in Chinook salmon in which a 4-day exposure to chlorpyrifos suppressed CYP1A protein levels (Table 7). However, even though both studies found OPs altered CYP1A protein levels in fish, these alterations were slight, and suggest that OPs are, at best, weak regulators of CYP1A protein in the fish studied. Rather, the main effect of OPs on CYP1A appears to be catalytic suppression (Flammarion et al., 1996, 1998; Paolini et al., 1997; Tang et al., 2002). Organophosphate effects on CYPs occur as a result of CYP-mediated metabolism, where OPs, such as chlorpyrifos, undergo desulfuration by CYPs leading to release of a free sulfur ion, which binds to the CYP heme and inhibits catalytic activity (Fukuto, 1990; Tang et al., 2002).
It is unlikely that reduced CYP1A protein expression in chlorpyrifos-treated fish would significantly affect chlorpyrifos toxicity. Induced CYP1A activity levels do not affect either the activation (via desulfuration) or detoxification (via dearylation) of chlorpyrifos in channel catfish (Straus et al., 2000). These findings, together with studies in humans demonstrating that other CYP isoforms (CYP2B6, CYP2C19, CYP3A4) are responsible for chlorpyrifos metabolism (Tang et al., 2001), suggest alterations in teleost CYP1A are unlikely to affect the toxicity of chlorpyrifos in fish.
5. Conclusion
This study showed that activity in two similar enzymes systems, carboxylesterase and AChE, was inhibited in vivo by exposure to chlorpyrifos. Levels of CYP1A were slightly suppressed at higher levels of chlorpyrifos, but esfenvalerate had no significant effect upon CYP1A levels or esterase activities. Individual variability in all three enzymes examined was fairly narrow, suggesting that it is appropriate to report enzyme activity from homogenates prepared from tissues of combined individuals. However, the small sample size (n = 10) may preclude the identification of individuals with decreased enzyme activity if it occurs with low frequency in the population. Of particular interest is the observation that solvent-exposed fish evidenced significant inhibition of carboxylesterase activity, even though the level of solvent was only 0.005%. This observation could have implications for in vivo studies on carboxylesterase activity. The use of multiple substrates to examine carboxylesterase activity showed substrate-specific responses, with PNPA detecting inhibition of activity at low chlorpyrifos doses that was not observed with other substrates. This observation combined with the lack of correlation between different esterase substrates suggests that it is necessary to use a battery of substrates when measuring carboxylesterase activity. Inhibition studies with splittail carboxylesterase activity further supported this finding, with IC50 values varying over several orders of magnitude for different substrates examined. The lack of observed hydrolysis activity of α-cyanoesters of pyrethroid acids for all fish species examined in this study suggests that these fish have very little esterase-mediated pyrethroid metabolism. This observation could account for some of the extreme sensitivity of fish to pyrethroid toxicity. Results indicated that Chinook salmon have multiple liver carboxylesterase isozymes, whose activity can be measured with a combination of substrates.
Carboxylesterase activity was inhibited at levels of chlorpyrifos that did not significantly affect AChE, suggesting a greater affinity of carboxylesterases than AChEs for some OPs. This observation supports the use of carboxylesterase activity as a biomarker of exposure to agrochemicals. The additional advantage of using carboxylesterase activity as a biomarker is its role in detoxifying pyrethroids. Therefore one measurement provides both a biomarker of exposure to OPs and/or carbamates as well as a biomarker of susceptibility to pyrethroid toxicity. It is likely that either marker alone would not provide enough information and given both the large amount of existing data on AChE levels as well as the ease of the assay, it is appropriate to continue monitoring the activity of this enzyme system. An advantage of using esterase activity (both carboxylesterase and AChE) as a reporter of OP exposure is that results are integrative. Exposure to multiple OPs should result in a concomitant decrease in enzyme activity, indicating total effects upon the exposed organism. However, there is little information in the literature on carboxylesterase activity and further work should attempt to determine constitutive levels in species important for biomonitoring projects. Carboxylesterase activity could serve as a key indicator of an organism’s exposure to agrochemicals or as a component of a comprehensive monitoring program to examine overall ecosystem health.
Acknowledgements
CEW was supported by NIH Post Doctoral training grant T32 DK07355-22 and a UC TSR & TP Graduate Fellowship. PDJ was supported by NIH Post Doctoral training grant T32 DK07355-22. This work was supported in part by the Calfed Ecosystem Restoration Program Project #99-N08, NIEHS Grant R37 ES02710, NIEHS Superfund Grant P42 ES04699, NIEHS Center for Environmental Health Sciences Grant P30 ES05707 and NIH/NIAID Grant U01 AI058267. The authors thank the Nimbus Fish Hatchery, the Center for Aquatic Biology and Aquaculture, the staff of the Aquatic Toxicology Laboratory, and Paul Lutes, John Henderson and Gina Lee. The CYP1A work was supported by the Department of the Interior, U.S. Geological Survey and the University of Kentucky Research Foundation, Grant Agreement No. 01HQGR0133, through the Kentucky Water Resources Research Institute. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Government.
References
- Abernathy CO, Casida JE. Pyrethroid insecticides: esterase cleavage in relation to selective toxicity. Science. 1973;179:1235–1236. doi: 10.1126/science.179.4079.1235. [DOI] [PubMed] [Google Scholar]
- Al-Ghais SM. Differential inhibition of xenobiotic-metabolizing carboxylesterases by organotins in marine fish. Ecotoxicol. Environ. Safety. 2000;46:258–264. doi: 10.1006/eesa.2000.1928. [DOI] [PubMed] [Google Scholar]
- Barron MG, Charron KA, Stott WT, Duvall SE. Tissue carboxylesterase activity of rainbow trout. Environ. Toxicol. Chem. 1999;18:2506–2511. [Google Scholar]
- Barry MJ, Ohalloran K, Logan DC, Ahokas JT, Holdway DA. Sublethal effects of esfenvalerate pulse-exposure on spawning and non-spawning Australian crimson-spotted rainbowfish (Melanotaenia-fluviatilis) Arch. Environ. Con. Tox. 1995;28:459–463. [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Bonacci S, Browne MA, Dissanayake A, Hagger JA, Corsi I, Focardi S, Galloway TS. Esterase activities in the bivalve mollusc Adamussium colbecki as a biomarker for pollution monitoring in the Antarctic marine environment. Mar. Pollut. Bull. 2004;49:445–455. doi: 10.1016/j.marpolbul.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Boone JS, Chambers JE. Biochemical factors contributing to toxicity differences among chlorpyrifos, parathion, and methyl parathion in mosquitofish (Gambusia affinis) Aquat. Toxicol. 1997;39:333–343. [Google Scholar]
- Bradbury SP, Coats JR. Comparative toxicology of the pyrethroid insecticides. Rev. Environ. Contam. Toxicol. 1989a;108:133–177. doi: 10.1007/978-1-4613-8850-0_4. [DOI] [PubMed] [Google Scholar]
- Bradbury SP, Coats JR. Toxicokinetics and toxicodynamics of pyrethroid insecticides in fish. Environ. Toxicol. Chem. 1989b;8:373–380. [Google Scholar]
- Brimijoin S, Koenigsberger C. Cholinesterases in neural development: new findings and toxicologic implications. Environ. Health Persp. 1999;107:59–64. doi: 10.1289/ehp.99107s159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchneli T, Fent K. Induction of cytochrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit. Rev. Environ. Sci. Technol. 1995;25:201–268. [Google Scholar]
- Bull CJ, McInerney JE. Behavior of juvenile coho salmon (Oncorhynchus-kisutch) exposed to Sumithion (fenitrothion), an organophosphate insecticide. J. Fish. Res. Board Can. 1974;31:1867–1872. [Google Scholar]
- Casida JE, Gammon DW, Glickman AH, Lawrence LJ. Mechanisms of selective action of pyrethroid insecticides. Annu. Rev. Pharmacol. Toxicol. 1983;23:413–438. doi: 10.1146/annurev.pa.23.040183.002213. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Metabolism and synergism of pyrethrins. In: Casida JE, Quistad GB, editors. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses. Oxford University Press; New York, NY: 1995. pp. 258–276. [Google Scholar]
- Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004;8:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Coppage DL, Matthews E. Brain acetylcholinesterase inhibition in a marine teleost during lethal and sublethal exposures to 1,2-dibromo-2,2-dichloroethyl dimethyl phosphate (Naled) in seawater. Toxicol. Appl. Pharmacol. 1975;31:128–133. doi: 10.1016/0041-008x(75)90060-5. [DOI] [PubMed] [Google Scholar]
- Coppage DL, Matthews E, Cook GH, Knight J. Brain acetylcholinesterase inhibition in fish as a diagnosis of environmental poisoning by malathion, O,O-dimethyl S-(1,2-dicarbethoxyethyl) phosphorodithioate. Pestic. Biochem. Physiol. 1975;5:536–542. [Google Scholar]
- Crepeau KL, Baker LM, Kuivila KM. Method of analysis and quality-assurance practices for determination of pesticides in water by solid-phase extraction and capillary-column gas chromatography/mass spectrometry at the U.S. Geological Survey California district organic chemistry laboratory, 1996-1999. U.S. Geological Survey Open-File Report 00-229. 2000. p. 19.
- DeKoven DL, Nunez JM, Lester SM, Conklin DE, Marty GD, Parker LM, Hinton DE. A purified diet for medaka (Oryzias latipes): refining a fish model for toxicological research. Lab. Anim. Sci. 1992;42:180–189. [PubMed] [Google Scholar]
- Denton DL, Wheelock CE, Murray S, Deanovic LA, Hammock BD, Hinton DE. Joint acute toxicity of esfenvalerate and diazinon to fathead minnow (Pimephales promelas) larvae. Environ. Toxicol. Chem. 2003;22:336–341. [PubMed] [Google Scholar]
- Draper NR, Hunter WG. Transformations: some examples revisited. Technometrics. 1969;11:23–40. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Elskus A, Pruell RJ, Stegeman JJ. Endogenously-mediated, pretranslational suppression of cytochrome P450IA expression in PCB-contaminated flounder. Mar. Environ. Res. 1992;34:97–101. [Google Scholar]
- Elskus AA. Estradiol and estriol suppress CYP1A expression in rainbow trout primary hepatocytes. Mar. Environ. Res. 2004;58:463–467. doi: 10.1016/j.marenvres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Escartin E, Porte C. The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ. Toxicol. Chem. 1997;16:2090–2095. [Google Scholar]
- Fent K, Bucheli TD. Inhibition of hepatic-microsomal monooxygenase system by organotins in-vitro in fresh-water fish. Aquat. Toxicol. 1994;28:107–126. [Google Scholar]
- Flammarion P, Migeon B, Garric J. Joint effects of copper sulphate and methidathion on rainbow trout (Oncorhynchus mykiss) EROD and AChE activities. Bull. Environ. Contam. Toxicol. 1996;56:440–445. doi: 10.1007/s001289900063. [DOI] [PubMed] [Google Scholar]
- Flammarion P, Migeon B, Urios SB, Morfin P, Garric J. Effect of methidathion on the cytochrome P-450 1A in the cyprinid fish gudgeon (Gobio gobio) Aquat. Toxicol. 1998;42:93–102. [Google Scholar]
- Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Persp. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton MH, Key PB. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 2001;20:37–45. doi: 10.1897/1551-5028(2001)020<0037:aiiefa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Galloway TS, Millward N, Browne MA, Depledge MH. Rapid assessment of organophosphorous/carbamate exposure in the bivalve mollusc Mytilus edulis using combined esterase activities as biomarkers. Aquat. Toxicol. 2002;61:169–180. doi: 10.1016/s0166-445x(02)00051-6. [DOI] [PubMed] [Google Scholar]
- Galloway TS, Brown RJ, Browne MA, Dissanayake A, Lowe D, Jones MB, Depledge MH. A multibiomarker approach to environmental assessment. Environ. Sci. Technol. 2004;38:1723–1731. doi: 10.1021/es030570+. [DOI] [PubMed] [Google Scholar]
- Gaughan LC, Engel JL, Casida JE. Pestcide interactions: effects of organophosphorus pesticides on the metabolism, toxicity, and persistence of selected pyrethroid insecticides. Pestic. Biochem. Physiol. 1980;14:81–85. [Google Scholar]
- Glickman AH, Hamid AA, Rickert DE, Lech JJ. Elimination and metabolism of permethrin isomers in rainbow trout. Toxicol. Appl. Pharmacol. 1981;57:88–98. doi: 10.1016/0041-008x(81)90028-4. [DOI] [PubMed] [Google Scholar]
- Glickman AH, Lech JJ. Hydrolysis of permethrin, a pyrethroid insecticide, by rainbow trout and mouse tissues in vitro: a comparative study. Toxicol. Appl. Pharmacol. 1981;60:186–192. doi: 10.1016/0041-008x(91)90222-z. [DOI] [PubMed] [Google Scholar]
- Glickman AH, Lech JJ. Differential toxicity of trans-permethrin in rainbow trout and mice. II. Role of target organ sensitivity. Toxicol. Appl. Pharmacol. 1982;66:162–171. doi: 10.1016/0041-008x(82)90281-2. [DOI] [PubMed] [Google Scholar]
- Glickman AH, Shono T, Casida JE, Lech JJ. In vitro metabolism of permethrin isomers by carp and rainbow trout liver microsomes. J. Agric. Food Chem. 1982;27:1038–1041. doi: 10.1021/jf60225a008. [DOI] [PubMed] [Google Scholar]
- Glickman AH, Weitman SD, Lech JJ. Differential toxicity of trans-permethrin in rainbow trout and mice. I. Role of biotransformation. Toxicol. Appl. Pharmacol. 1982;66:153–161. doi: 10.1016/0041-008x(82)90280-0. [DOI] [PubMed] [Google Scholar]
- Goksoyr A. Use of cytochrome P450 1A (CYP1A) in fish as a biomarker of aquatic pollution. Arch. Toxicol. Suppl. 1995;17:80–95. doi: 10.1007/978-3-642-79451-3_7. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Dettbarn WD. Role of carboxylesterases in the prevention and potentiation of N-methylcarbamate toxicity. Chem. Biol. Interact. 1993;87:295–303. doi: 10.1016/0009-2797(93)90057-6. [DOI] [PubMed] [Google Scholar]
- Heder AF, Hirsch-Ernst KI, Bauer D, Kahl GF, Desel H. Induction of cytochrome P4502B1 by pyrethroids in primary rat hepatocyte cultures. Biochem. Pharmacol. 2001;62:71–79. doi: 10.1016/s0006-2952(01)00639-6. [DOI] [PubMed] [Google Scholar]
- Huang TL, Obih PO, Jaiswal R, Hartley WR, Thiyagarajah A. Evaluation of liver and brain esterases in the spotted gar fish (Lepisosteus oculatus) as biomarkers of effect in the lower Mississippi River Basin. Bull. Environ. Contam. Toxicol. 1997;58:688–695. doi: 10.1007/s001289900388. [DOI] [PubMed] [Google Scholar]
- Huang TL, Szekacs A, Uematsu T, Kuwano E, Parkinson A, Hammock BD. Hydrolysis of carbonates, thiocarbonates, carbamates, and carboxylic esters of α-naphthol, β-naphthol, and p-nitrophenol by human, rat, and mouse liver carboxylesterases. Pharm. Res. 1993;10:639–648. doi: 10.1023/a:1018987111362. [DOI] [PubMed] [Google Scholar]
- Hunt JW, Anderson BS, Phillips BM, Nicely PN, Tjeerdema RS, Puckett HM, Stephenson M, Worcester K, De Vlaming V. Ambient toxicity due to chlorpyrifos and diazinon in a central California coastal watershed. Environ. Monit. Assess. 2003;82:83–112. doi: 10.1023/a:1021677914391. [DOI] [PubMed] [Google Scholar]
- Keizer J, D’Agostino G, Nagel R, Volpe T, Gnemi P, Vittozzi L. Enzymological differences of AChE and diazinon hepatic metabolism: correlation of in vitro data with the selective toxicity of diazinon to fish species. Sci. Total Environ. 1995;171:213–220. doi: 10.1016/0048-9697(95)04687-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Miyagaki T, Wakabayashi M. Evaluation of pesticides used in golf links by acute toxicity test on rainbow trout. Bull. Jpn. Soc. Sci. Fish. 1996;62:414–419. [Google Scholar]
- Kingsbury N, Masters CJ. Heterogeneity, molecular weight interrelationships and developmental genetics of the esterase isoenzymes of the rainbow trout. Biochim. Biophys. Acta. 1972;258:455–465. doi: 10.1016/0005-2744(72)90237-9. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ. Health Persp. 1999;107:65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist A, Augustinsson KB. Purification and properties of two carboxylesterases from rat-liver microsomes. Eur. J. Biochem. 1971;23:303–313. doi: 10.1111/j.1432-1033.1971.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maxwell DM. The specificity of carboxylesterase protection against the toxicity of organophosphate compounds. Toxicol. Appl. Pharmacol. 1992;114:306–312. doi: 10.1016/0041-008x(92)90082-4. [DOI] [PubMed] [Google Scholar]
- McArdle ME, McElroy AE, Elskus AA. CYP1A, CYP3A and estradiol 2-hydroxylase in fish exposed to organic pollutants and sewage effluent. Environ. Toxicol. Chem. 2004;23:953–959. doi: 10.1897/03-82. [DOI] [PubMed] [Google Scholar]
- McKim JM, Bradbury SP, Niemi GJ. Fish acute toxicity syndromes and their use in the QSAR approach to hazard assessment. Environ. Health Persp. 1987;71:171–186. doi: 10.1289/ehp.8771171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill AJ, Galloway TS, Browne MA, Dissanayake A, Depledge MH. Evaluation of toxicity in tributaries of the Mersey estuary using the isopod Asellus aquaticus (L.) Mar. Environ. Res. 2004;58:327–331. doi: 10.1016/j.marenvres.2004.03.076. [DOI] [PubMed] [Google Scholar]
- Paolini M, Pozzetti L, Sapone A, Mesirca R, Perocco P, Mazzullo M, Cantelli-Forti G. Molecular non-genetic biomarkers of effect related to acephate cocarcinogenesis: sex- and tissue-dependent induction or suppression of murine CYPs. Cancer Lett. 1997;117:7–15. doi: 10.1016/s0304-3835(97)00177-8. [DOI] [PubMed] [Google Scholar]
- Parker LM, Lauren DJ, Hammock BD, Winder B, Hinton DE. Biochemical and histochemical properties of hepatic tumors of rainbow trout, Oncorhynchus mykiss. Carcinogenesis. 1993;14:211–217. doi: 10.1093/carcin/14.2.211. [DOI] [PubMed] [Google Scholar]
- Phillips BM, Anderson BS, Hunt JW, Nicely PA, Kosaka RA, Tjeerdema RS, de Vlaming V, Richard N. In situ water and sediment toxicity in an agricultural watershed. Environ. Toxicol. Chem. 2004;23:435–442. doi: 10.1897/03-93. [DOI] [PubMed] [Google Scholar]
- Phipps GL, Holcombe GW. A method for aquatic multiple species toxicant testing acute toxicity of 10 chemicals to 5 vertebrates and 2 invertebrates. Environ. Pollut. A. 1985;38:141–158. [Google Scholar]
- Poet TS, Wu H, Kousba AA, Timchalk C. In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol. Sci. 2003;72:193–200. doi: 10.1093/toxsci/kfg035. [DOI] [PubMed] [Google Scholar]
- Post G, Leasure RA. Sublethal effect of malathion to three salmonid species. Bull. Environ. Contam. Toxicol. 1974;12:312–319. doi: 10.1007/BF01709125. [DOI] [PubMed] [Google Scholar]
- Rickwood CJ, Galloway TS. Acetylcholinesterase inhibition as a biomarker of adverse effect. A study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos. Aquat. Toxicol. 2004;67:45–56. doi: 10.1016/j.aquatox.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Riddles PW, Schnitzerling HJ, Davey PA. Application of trans and cis isomers of p-nitrophenyl-(1R,S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate to the assay of pyrethroid hydrolyzing esterases. Anal. Biochem. 1983;132:105–109. doi: 10.1016/0003-2697(83)90431-1. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit. Rev. Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Sanchez-Hernandez JC, Fossi MC, Leonzio C, Focardi S. Use of biochemical biomarkers as a screening tool to focus the chemical monitoring of organic pollutants in the Biobio River basin (Chile) Chemosphere. 1998;37:699–710. [Google Scholar]
- Sandahl JF, Jenkins JJ. Pacific steelhead (Oncorhynchus mykiss) exposed to chlorpyrifos: Benchmark concentration estimates for acetylcholinesterase inhibition. Environ. Toxicol. Chem. 2002;21:2452–2458. [PubMed] [Google Scholar]
- SAS . Procedures Guide for Personal Computers. SAS Institute; Cary, NC: 1985. 6th ed. [Google Scholar]
- Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu. Rev. Pharmacol. Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- Scott JG. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. 1999;29:757–777. doi: 10.1016/s0965-1748(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Segel IH. Biochemical Calculations. John Wiley & Sons Inc.; New York: 1976. p. 441. 2nd ed. [Google Scholar]
- Shan G, Hammock B. Development of sensitive esterase assays based on α-cyano-containing esters. Anal. Biochem. 2001;299:54–62. doi: 10.1006/abio.2001.5388. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 2002;128:215–228. doi: 10.1016/s0378-4274(01)00543-4. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Hahn ME. Biochemistry and molecular biology of monooxygenases: current perspectives on forms, functions, and regulation of cytochrome P450 in aquatic species. In: Malins DC, Ostrander GK, editors. Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives. Lewis; Ann Arbor: 1994. pp. 87–206. [Google Scholar]
- Stegeman JJ, Lech JJ. Cytochrome P-450 monooxygenase systems in aquatic species: carcinogen metabolism and biomarkers for carcinogen and pollutant exposure. Environ. Health Persp. 1991;90:101–109. doi: 10.1289/ehp.90-1519513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stok J, Huang H, Jones PD, Wheelock CE, Morisseau C, Hammock BD. Identification, expression and purification of a pyrethroid hydrolyzing carboxylesterase from mouse liver microsomes. J. Biol. Chem. 2004;279:29863–29869. doi: 10.1074/jbc.M403673200. [DOI] [PubMed] [Google Scholar]
- Straus DL, Schlenk D, Chambers JE. Hepatic microsomal desulfuration and dearylation of chlorpyrifos and parathion in fingerling channel catfish: lack of effect from Aroclor 1254. Aquat. Toxicol. 2000;50:141–151. doi: 10.1016/s0166-445x(99)00088-0. [DOI] [PubMed] [Google Scholar]
- Sturm A, Wogram J, Segner H, Liess M. Different sensitivity to organophosphates of acetylcholinesterase and butyrlcholinesterase from three-spined stickleback (Gasterosteus aculeatus) application in biomonitoring. Environ. Toxicol. Chem. 2000;19:1607–1615. [Google Scholar]
- Symons PEK. Behavior of young Atlantic salmon (Salmo salar) exposed to or force-fed fenitrothion, an organophosphate insecticide. J. Fish. Res. Board Can. 1973;30:651–655. [Google Scholar]
- Tang J, Cao Y, Rose RL, Brimfield AA, Dai D, Goldstein JA, Hodgson E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab. Dispos. 2001;29:1201–1204. [PubMed] [Google Scholar]
- Tang J, Cao Y, Rose RL, Hodgson E. In vitro metabolism of carbaryl by human cytochrome P450 and its inhibition by chlorpyrifos. Chem. Biol. Interact. 2002;141:229–241. doi: 10.1016/s0009-2797(02)00074-1. [DOI] [PubMed] [Google Scholar]
- U.S. EPA . Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. Environmental Protection Agency; 1985. pp. 58–75. EPA/600/4/85/014. [Google Scholar]
- U.S. EPA . Pesticide Ecotoxicity Database. (Formerly: Environmental Effects Database (EEDB)); 2000. [Google Scholar]
- Van Dolah RF, Maier PP, Fulton MH, Scott GI. Comparison of azinphosmethyl toxicity to juvenile red drum (Sciaenops ocellatus) and the mummichog (Fundulus heteroclitus) Environ. Toxicol. Chem. 1997;16:1488–1493. [Google Scholar]
- Werner I, Deanovic LA, Connor V, de Vlaming V, Bailey HC, Hinton DE. Insecticide-caused toxicity to Ceriodaphnia dubia (Cladocera) in the Sacramento-San Joaquin River Delta, California, USA. Environ. Toxicol. Chem. 2000;19:215–227. [Google Scholar]
- Werner I, Deanovic LA, Hinton DE, Henderson JD, de Oliveira GH, Wilson BW, Krueger W, Wallender WW, Oliver MN, Zalom FG. Toxicity of stormwater runoff after dormant spray application of diazinon and esfenvalerate (Asana) in a French prune orchard, Glenn county, California, USA. Bull. Environ. Contam. Toxicol. 2002;68:29–36. doi: 10.1007/s00128-001-0215-7. [DOI] [PubMed] [Google Scholar]
- Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD. Development of toxicity identification evaluation (TIE) procedures for pyrethroid detection using esterase activity. Environ. Toxicol. Chem. 2004;11:2699–2708. doi: 10.1897/03-544. [DOI] [PubMed] [Google Scholar]
- Wheelock CE, Severson TF, Hammock BD. Synthesis of new carboxylesterase inhibitors and evaluation of potency and water solubility. Chem. Res. Toxicol. 2001;14:1563–1572. doi: 10.1021/tx015508+. [DOI] [PubMed] [Google Scholar]
- Wheelock CE, Wheelock AM, Å Zhang R, Stok JE, Le Valley SE, Green CE, Hammock BD. Evaluation of α-cyanoesters as fluorescent substrates for examining interindividual variation in general and pyrethroid-selective esterases in human liver microsomes. Anal. Biochem. 2003;315:208–222. doi: 10.1016/s0003-2697(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Wheelock CE, Shan G, Ottea J. Overview of carboxylesterases and their role in the metabolism of insecticides. J. Pestic. Sci. 2005;30:75–83. [Google Scholar]
- Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000;30:347–570. doi: 10.1080/10408440091159239. [DOI] [PubMed] [Google Scholar]
- Wildish DJ, Lister NA. Biological effects of fenitrothion in the diet of brook trout. Bull. Environ. Contam. Toxicol. 1973;10:333–339. doi: 10.1007/BF01720999. [DOI] [PubMed] [Google Scholar]
- Wogram J, Sturm A, Segner H, Liess M. Effects of parathion on acetylcholinesterase, butyrylcholinesterase, and carboxylesterase in three-spined stickleback (Gasterosteus aculeatus) following short-term exposure. Environ. Toxicol. Chem. 2001;20:1528–1531. doi: 10.1897/1551-5028(2001)020<1528:eopoab>2.0.co;2. [DOI] [PubMed] [Google Scholar]