Abstract
Introduction
The rising incidence of hepatocellular carcinoma (HCC) in western countries, along with the poor prognosis offered by present-day treatment modalities, makes novel therapies for this disease necessary. Oncolytic herpes simplex viruses (HSV) are replication-competent viruses that are highly effective in the treatment of a wide variety of experimental models of human malignancies. This study seeks to investigate the effectiveness of oncolytic herpes viruses in the treatment of primary hepatocellular carcinoma cell lines.
Methods
Sixteen commercially available human hepatocellular carcinoma cell lines were studied. G207 is an attenuated, replication-competent, oncolytic herpes simplex virus (HSV) engineered to selectively replicate within cancer cells. Cell lines were tested for viral sensitivity to G207 and their ability to support viral replication using standard cytotoxicity and viral replication assays.
Results
Eleven of sixteen cell lines were moderately-to-highly sensitive to G207 viral oncolysis. HCC cell lines additionally demonstrated the ability to support viral replication in vitro with as high as 800-fold amplification of the administered viral dose observed.
Conclusions
G207 is cytotoxic to, and efficiently replicates within, HCC cell lines in vitro. From these data, we suggest that oncolytic HSV therapy may have a role in the treatment of HCC and in vivo studies are warranted.
Keywords: Hepatocellular carcinoma, G207, Herpes simplex virus, Gene therapy
Introduction
Hepatocellular carcinoma is one of the most common malignancies worldwide with a global annual incidence of nearly 1 million cases(1–3). While the incidence throughout Asia and Africa remains relatively stable, western countries are experiencing a rise in the incidence of this disease. The American Cancer Society surveillance data estimated 18 920 new cases in 2004, representing a significant rise from previous years(4). It was also estimated that 14 270 deaths occurred as a result of the disease in 2004. While surgical resection presently remains the only curative option for this disease, the majority of patients who undergo resection eventually succumb to the disease. It is clear that novel therapies are needed for the treatment of this aggressive cancer.
Oncolytic herpes simplex viruses (HSV) have recently been shown to be effective in the treatment of a wide variety of human malignancies in animal models including brain, colorectal, lung, gastric, prostate, breast, bladder and head and neck cancers(5–17). These attenuated, replication-competent viruses present an exciting new treatment modality in cancer therapy through their ability to selectivity replicate within cancer cells while sparing normal cells(10). This study examines the effectiveness of oncolytic herpes viruses in the treatment of primary hepatocellular carcinoma cell lines in vitro. As such, it provides a framework for in vivo studies investigating the use of an oncolytic herpes virus in the treatment of primary hepatocellular carcinoma. In doing so, we have also established a comprehensive compilation characterizing all known human hepatocellular carcinoma cell lines.
Materials and methods
Cell Lines
All known commercially available human hepatocellular carcinoma cell lines were studied. Hep 3B2.1–7 (Hep3B, Hep 3B, Hep-3B, HB-8064), Hep G2 (HepG2, HB-8065), PLC/PRF/5 (PLC5, CRL-8024) and SK-HEP-1(HTB-52) cell lines were obtained from the American Type Culture Collection (ATCC®, Manassas, VA, USA). They were grown in vitro in ATCC medium with phenol red control [Eagle’s Minimum essential medium (MEM) with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5 g/L sodium bicarbonate, containing 0.1 mM non-essential amino acids(NEAA), and 1.0 mM sodium pyruvate, 100 U/ml penicillin and 100 mg/ml streptomycin with 10% fetal calf serum (FCS, Atlanta Biologicals, Norcross, GA, USA)]. An additional 12 human hepatocellular carcinoma cell lines (SNU-182, SNU-354, SNU-368, SNU-387, SNU-398, SNU-423, SNU-449, SNU-475, SNU-739, SNU-761, SNU-878 and SNU-886) were obtained from the Korean Cell Line Bank (KCLB, Seoul, South Korea) and were grown in phenol red controlled RPMI 1640 with 20 mM HEPES, 2.0 g/L sodium bicarbonate, 100 U/ml penicillin and 100mg/ml streptomycin, and 10% FCS. African green monkey kidney cells (Vero cells, ATCC®, Manassas, VA, USA) for viral plaque assays were grown in MEM containing 10% FCS and 100 U/ml penicillin and 100mg/ml streptomycin. All cells were maintained in a 5% CO2 humidified incubator at 37°C.
Virus
G207 is an attenuated, replication-competent, oncolytic herpes simplex virus type-1 (HSV-1) whose construction has been previously described in detail(14;18). G207, a second-generation virus derived from R3616, a construct by Dr. Roizman, is attenuated by the deletion of a single copy of the diploid ICP6 gene and deletion of both copies of the γ1 34.5 neurovirulence gene(14;18–20). The virus also contains an insertion of the E.coli β-galactosidase (LacZ) gene into the UL39 gene which serves as a marker of an infection(14;18). Disruption of the UL39 gene eliminates ribonucleotide reductase (RR) activity and increases specificity of the virus for proliferating cells such as tumor cells(18;21–23). G207 was titered by standard plaque assay as previously described(14;17;18).
Cytotoxicity Assay
All hepatocellular carcinoma cell lines were plated at a density of 1x104 cells per well in 12-well flat-bottom plates (Costar, Corning Inc., Corning, NY, USA) in 1 ml of media. After incubation for 6 hours, G207 (diluted in 50 μL phosphate buffered saline (PBS)) was added to each well at a multiplicity of infection (MOI, the ratio of viral plaque forming units (pfu) per tumor cell) of three dilutions ranging from 0.01 to 3.0. On days 1,3,5,7, and 9 after infection, supernatant samples were collected and frozen, and wells were washed with PBS. Cells were then lysed with Triton-X (1.35%) to release intracellular lactate dehydrogenase (LDH). LDH was quantified using a Cytotox 96 non-radioactive cytotoxicity assay (Promega, Madison, WI, USA) which is a colorimetric analysis measuring the conversion of a tetrazolium salt into a red formazan product in the presence of LDH(24–26). The amount of color formed is directly proportional to the number of lysed cells. Absorbance was measured at 450 nm using a microplate spectrophotometer (EL312e, Bio-Tek Instruments, Winooski VT, USA). Results are expressed as the surviving fraction of cells as determined by the measured absorbance of each sample relative to control, untreated, cell lysates. All samples were analyzed in triplicate and each experiment was repeated in triplicate to ensure reproducibility.
Viral Replication Assay
Supernatant samples were collected and frozen from all wells of the cytotoxicity (MOI 0.01, 0.1 and 1.0) experiments (days 1–8) after infection with G207. Vero cells were grown to confluence on 6-well flat-bottom plates (Costar, Corning, Inc., Corning, NY, USA). Supernatant samples were thawed, and serial 10-fold dilutions of the samples were incubated on Vero cells for 4 hours. Wells were then gently washed with media and covered with 1% agarose with media. After 48 hours of incubation, 2 ml of neutral red solution was added and after an additional 24 hours of incubation, viral plaques were counted. All samples were analyzed in triplicate.
Tumorigenicity Assay
Six-week old athymic nude mice (National Cancer Institute, Bethesda, MD, USA) were used to assess tumorigenicity of all hepatocellular carcinoma cell lines. All animal procedures were performed with the approval of the Memorial Sloan-Kettering Institutional Animal Care and Use Committee. Animals were anesthetized with inhalational isoflurane for all experimental procedures. Flank tumors were established by injection of 1x107 tumor cells in 50μL of PBS into the subcutaneous flanks of athymic nude mice. Tumor development and growth was assessed by measuring tumor dimensions three times a week. Animals were weighed and monitored closely for signs of distress including poor grooming, cachexia, respiratory distress, cutaneous ulcerations, or tumor progression. Animals without any evidence of tumor progression or toxicity were monitored for up to 3 months.
Results
Cell Lines
The hepatocellular carcinoma cell lines Hep3B, HepG2, PLC5 and SK-HEP-1 (ATCC) and SNU-182, SNU-354, SNU-368, SNU-387, SNU-398, SNU-423, SNU-449, SNU-475, SNU-739, SNU-761, SNU-878 and SNU-886 (KCLB) grew readily in culture. Previously determined characteristics of these cell lines including origin, morphology, HBV gene integration, HBx DNA status, cellular products and protein expression are listed in Table I(27–41).
Table 1.
| Cell Bank | |||||
|---|---|---|---|---|---|
| Generic name | Origin | Cell shape in culture | HBV gene integration | Biosafety level | Cellular products and protein expression |
| Growth morphology | HBx DNA detect | Tumorigenicity | |||
| ATCC | |||||
| Hep 3B | human, HCC, black male | polygonal, epithelial | Yes | II | HBsAg, HBx protein, alpha-FP, albumin, alpha-2-macroglobulin, alpha 1 antitrypsin, transferrin, haptoglobulin, alpha 1 antichymotrypsin, celuloplasmin, plasminogen, C3, C4, C3 activator, fibrinogen, alpha 1 acid glycoprotein, alpha 2 HS glycoprotein, beta lipoprotein, retinol binding protein, Gc globulin |
| monolayer, adherent | Yes | Yes | |||
| Hep G2 | human, HCC, Caucasian male | polygonal-round, epithelial | No | I | alpha-FP, albumin, alpha-2-macroglobulin, alpha 1 antitrypsin, transferrin, alpha 1 antichymotrypsin, haptoglobulin, celuloplasmin, plasminogen, C4, C3 activator, fibrinogen, alpha 1 acid glycoprotein, alpha 2 HS glycoprotein, beta lipoprotein, retinol binding protein, IGF-II, 3-hydroxy-3methylglutaryl CoA reductase, hepatic triglyceride lipase |
| multilayer hump, adherent | No | No | |||
| SK-Hep-1 | human, HCC, Caucasian male | spindle-round, epithelial | No | I | alpha 1 antitrypsin, IGF-binding protein 3 |
| monolayer, adherent | No | Yes | |||
| PLC/PRF/5 | human, HCC, black male | polygonal | Yes | II | HBsAg, alpha-FP |
| monolayer, adherent | No | Yes | |||
| KCLB | |||||
| SNU-182 | human, HCC, Asian male | polygonal | Yes | II | HBsAg |
| monolayer, adherent | Yes | Yes | |||
| SNU-354 | human, HCC, Asian male | polygonal | Yes | II | HBsAg, HBx protein,MDR1 gene, albumin |
| monolayer, adherent | Yes | No | |||
| SNU-368 | human, HCC, Asian male | polygonal | Yes | II | HBsAg,HBx protein,MDR1 gene, tranferrin,IGF-II |
| monolayer, adherent | Yes | Yes | |||
| SNU-387 | human, hepatocellular carcinoma, Asian female | polygonal-spindle | Yes | II | HBsAg |
| monolayer, adherent | Yes | Yes | |||
| SNU-398 | human, HCC, Asian male | round-spindle | Yes | II | HBsAg |
| monolayer, adherent, floating | Yes | Yes | |||
| SNU-423 | human, HCC, Asian male | polygonal-spindle | Yes | II | HBsAg |
| monolayer, adherent | No | Yes | |||
| SNU-449 | human, HCC, Asian male | polygonal | Yes | II | HBsAg |
| monolayer, adherent | Yes | Yes | |||
| SNU-475 | human, HCC, Asian male | polygonal-round | Yes | II | HBsAg |
| monolayer, adherent | Yes | No | |||
| SNU-739 | human, HCC, Asian male | spindle | Yes | II | HBsAg,HBcAg,HBx protein |
| monolayer, adherent | Yes | No | |||
| SNU-761 | human, HCC, Asian male | polygonal | Yes | II | HBsAg,HBcAg,HBx protein, albumin |
| monolayer, adherent | Yes | No | |||
| SNU-878 | human, HCC, Asian female | polygonal | Yes | II | HBsAg,HBcAg,HBx protein, albumin, transferrin |
| monolayer, adherent | Yes | Yes | |||
| SNU-886 | human, HCC, Asian male | polygonal-spindle | Yes | II | HBsAg,HBcAg,HBx protein, albumin, transferrin |
| monolayer, adherent | Yes | Yes | |||
Viral Cytotoxicity
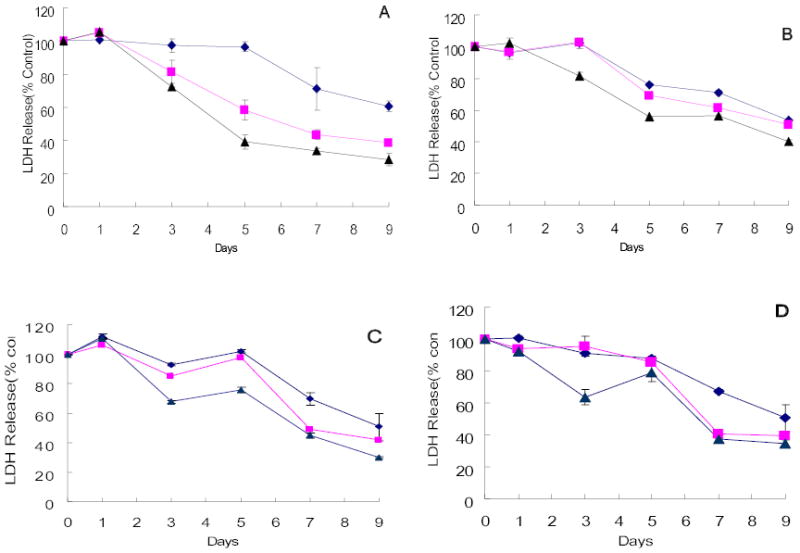
The ability of G207 to kill hepatocellular carcinoma cell lines was assessed. Three of the four cell lines obtained from the ATCC were sensitive to G207 cytotoxicity. HepG2 and PLC5 demonstrated nearly 100% cell kill by day 7 after infection at an MOI of 1.0 (Figure 1A–B). At an MOI of 0.5, Hep3B similarly showed near-complete cell kill (Figure 1C). Even at an MOI of 0.1, these three cell lines demonstrated 93.0%, 83.7%, 83.8% cytotoxicity by day 7, respectively (HepG2, PLC5, Hep3B). In contrast, only SK-HEP-1 was resistant to viral infection with no significant cytotoxicity demonstrated at an MOI as high as 3.0 (Figure 1D).
Figure 1.
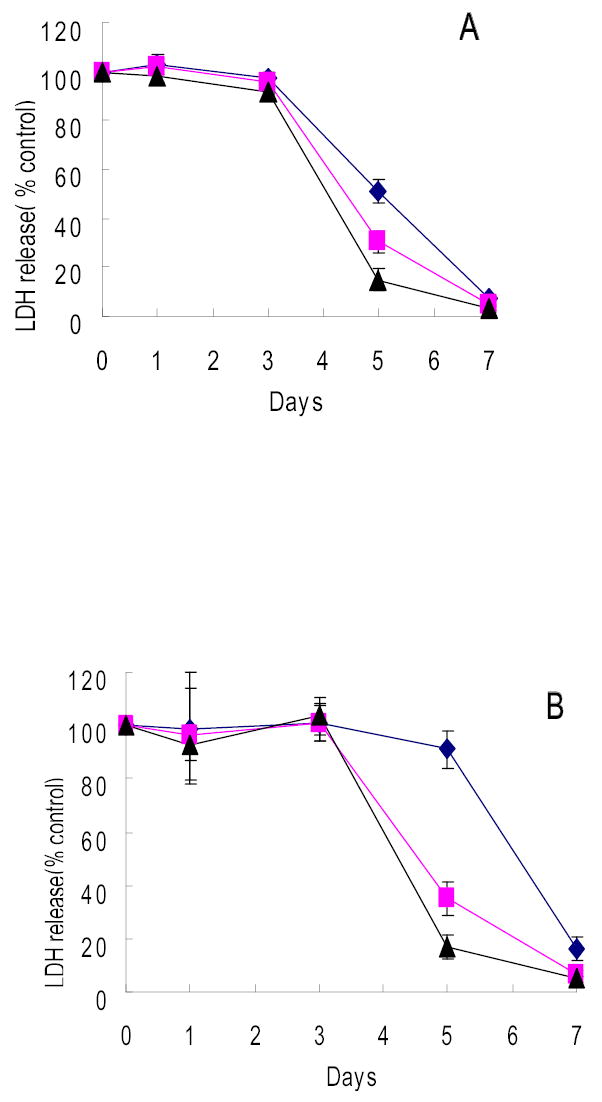
Cytotoxicity of G207 against hepatocellular carcinoma cell lines of ATCC origin in vitro. HCC cell lines HepG2(A), PLC/PRF/5(B), and Hep3B(C) were sensitive to G207 viral oncolysis whereas SK-HEP-1 (D) was not sensitive. Doses of virus used were MOI=0.1(diamond), MOI=0.5(square), MOI=1(triangle). MOI represents the ratio of the number of viral particles to the number of tumor cells.
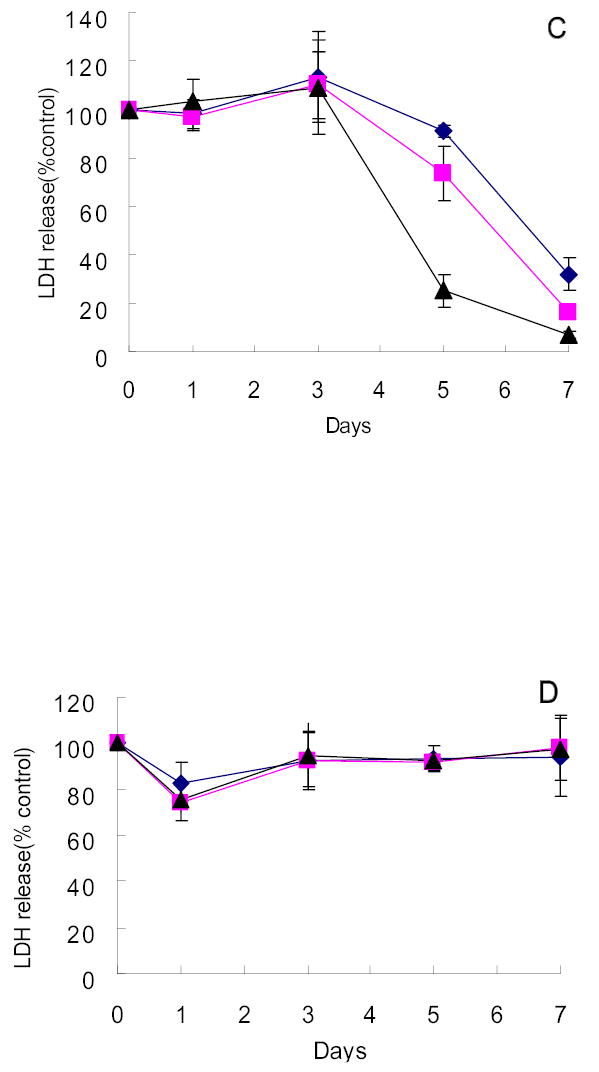
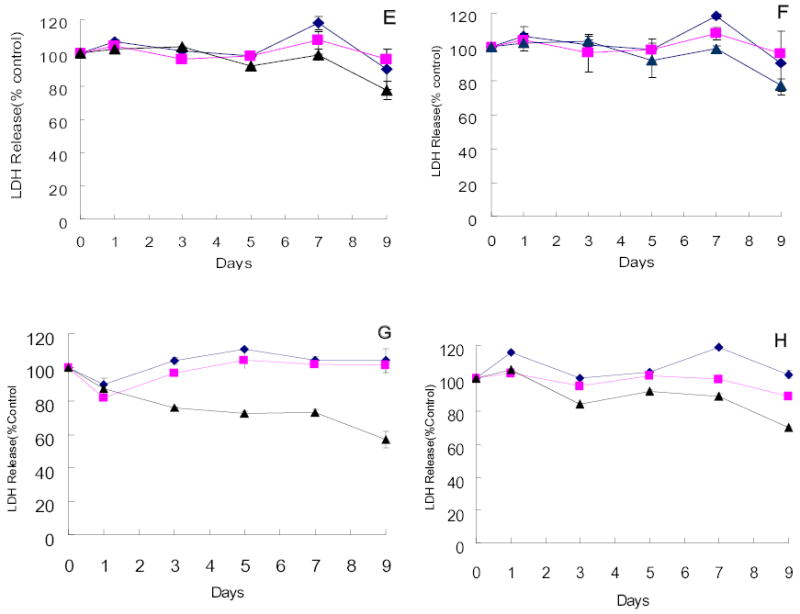
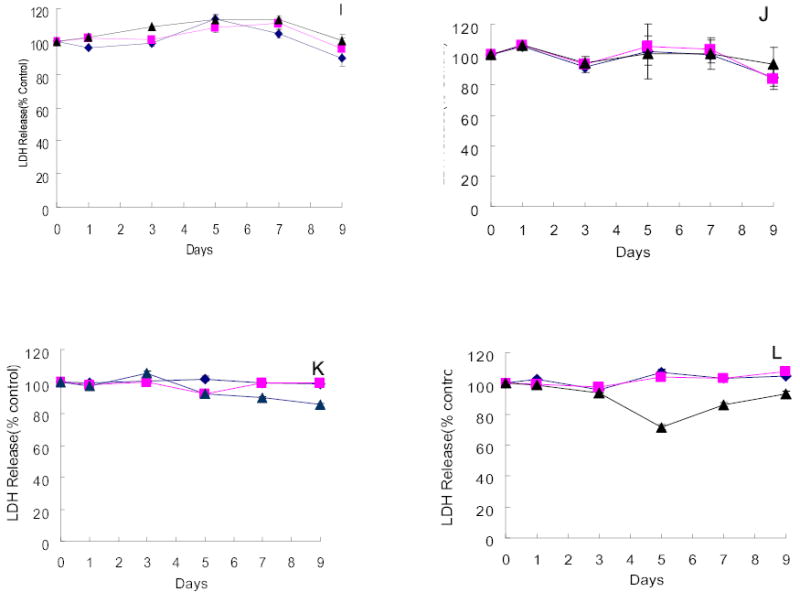
Four of the twelve hepatocellular carcinoma cell lines obtained from the KCLB were highly sensitive to G207 cytotoxicity. SNU-182, SNU-387, SNU-886 and SNU-878 demonstrated 71.5%, 59.8%, 65.5%, and 70.4% cell kill by day 9 after infection at an MOI of 1.0, respectively (Figure 2A–D). Even at an MOI of 0.01, only 60.6%, 53.4%, 50.4%, and 50.6% of cells remained viable by day 9 after infection, respectively. Four cell lines demonstrated moderate sensitivity to viral infection. At an MOI of 1.0, SNU-368, SNU-449, SNU-739 and SNU-761 showed 22.4%, 22.4%, 43.2%, and 29.9% cell kill, respectively (Figure 2E–H). In contrast, the remaining four cell lines of KCLB origin (SNU-398, SNU-475, SNU-354 and SNU-423) were resistant to viral infection with no significant cytotoxicity demonstrated 9 days after infection (Figure 2I–L).
Figure 2.
Cytotoxicity of G207 against hepatocellular carcinoma cell lines of KCLB origin in vitro. Four cell lines, SNU-182(A), SNU-387(B), SNU-878(C) and SNU-886(D) were highly sensitive to viral oncolysis and four cell lines, SNU-368(E), SNU-449(F), SNU-739(G) and SNU-761(H), demonstrated moderate sensitivity. SNU-354(I), SNU-398(J), SNU-423(K), and SNU-475(L) showed minimal sensitivity to on the viral oncolysis. Doses of virus used were MOI=0.01(diamond), MOI=0.1(square), MOI=1(triangle). MOI represents the ratio of the number of viral particles to the number of tumor cells.
Viral Replication
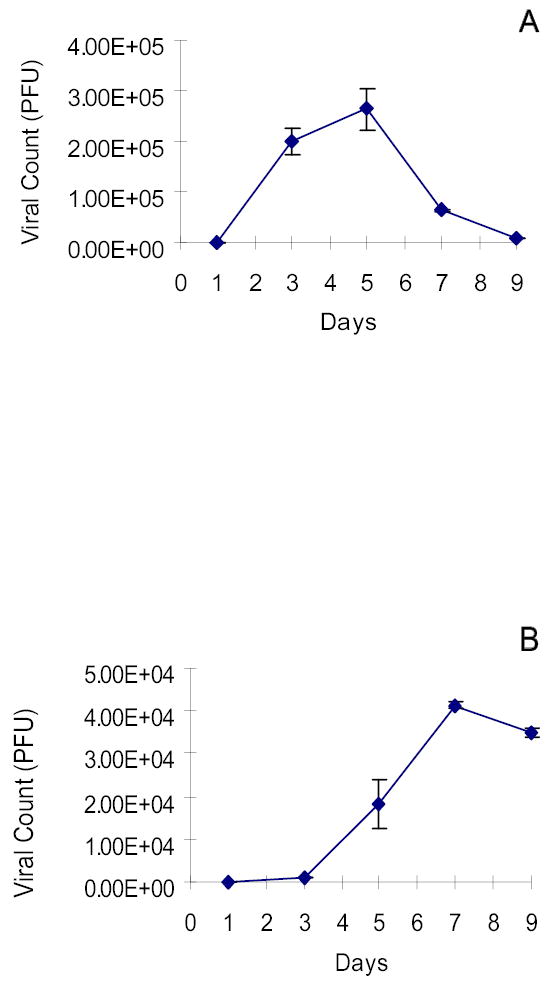
The ability of G207 to replicate in human hepatocellular carcinoma cell lines was also demonstrated. Representative data is shown in Figure 2. Hep3B strongly supported logarithmic viral proliferation with peak viral titers measuring 2.5x105 pfu 5 days after infection with G207 at an MOI of 0.01 (3x102 pfu) (Figure 3A). This represents a greater than 800-fold amplification of the initial viral dose after 5 days. HepG2 and PLC5 also supported G207 viral replication 7 days after infection at an MOI of 0.1 (3x103 pfu), demonstrating peak infectious virion recovery of 5x104 pfu and 2.5x104 pfu, respectively (Figure 3B–C).
Figure 3.
Viral replication is supported by HCC cell lines. Viral plaque assay demonstrates high viral yield in Hep3B(A), HepG2(B) and PLC/PRF/5(C) following infection with G207. Hep3B most strongly supported viral proliferation with peak viral titers measuring 2.5x105 pfu 5 days after infection with G207 (3x102 pfu) representing a greater than 800-fold amplification of the initial viral dose. Doses of virus used were MOI=0.01 for Hep3B, MOI=0.1 for HepG2 and PLC5. MOI represents the ratio of the number of viral particles to the number of tumor cells.
Tumorigenicity
Tumorigenicity of all sixteen hepatocellular carcinoma cell lines was examined and is listed in Table I(27–41). The ability of Hep3B, PLC5, and SK-Hep1 to form tumors in nude athymic mice was already known from previous reports. Of the ATCC-obtained cell lines, only HepG2 does not form tumors in nude mice. Eight of twelve cell lines of KCLB origin (SNU-182, SNU-368, SNU-387, SNU-398, SNU-423, SNU-449, SNU-878 and SNU-886) produced flank tumors within two weeks of tumor cell implantation into nude mice, with tumor diameters ranging from 3mm to 18mm. The remaining four cell lines (SNU-354, SNU-475 SNU-739 and SNU-761) failed to develop tumors as long as three months after implantation.
Discussion
Primary hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide(1–3). There are clear geographic epidemiologic differences with the highest incidences seen in Asia and Africa(3;42;43). Western countries, however, are experiencing a surge in the incidence of this disease. In the United States, the incidence of HCC increased from 1.4 per 100,000 population in 1980 to 2.4 per 100,000 population in 1995. It further increased to 6.7 per 100,000 population for men and 3.1 for women in the year 2000(44;45). In 2004, American Cancer Society surveillance data estimated 18,920 new cases and 14,270 deaths due to HCC(4). Major risk factors for the development of hepatocellular carcinoma include chronic hepatitis B and C infection and alcohol consumption, and it is an increase in incidence of these factors that are thought to be responsible for this rise.
In a recent review, median survival of patients who underwent curative resection for hepatocellular carcinoma was only 39 months, with 1- and 3-year survival rates of 81% and 54%, respectively(46). In this series, patients who underwent tumor ablation demonstrated a median survival of 15 months with 1- and 3-year survival rates of 56% and 21%, respectively(46). Patients treated with systemic chemotherapy or supportive care demonstrated a median survival of only 9 months, with a 44% 1-year survival rate and no 3-year survivors(46).
While hepatic resection remains the best curative option, there are many instances where curative resection cannot be performed including advanced patient age, underlying severe comorbidities or poor liver function, and locally advanced disease. While numerous alternative treatment modalities that have been employed in this setting including percutaneous microwave coagulation therapy (PMCT), percutaneous ethanol infusion (PEI), regional hyperthermia therapy, interstitial radiotherapy and radiofrequency interstitial tissue ablation (RITA, RFA), none have yet been demonstrated to improve patient survival(47–53). It is clear that novel therapies are needed.
Attenuated, replication-competent oncolytic herpes simplex viruses offer an exciting new modality in the armamentarium of anti-cancer agents. A wealth of recent experiments has demonstrated the effectiveness of these oncolytic herpes viruses in the treatment of a wide variety of human cancer cell lines. We now show that the majority of all known human hepatocellular carcinoma cell lines are also sensitive to treatment with an oncolytic herpes virus, G207. Eleven of sixteen cell lines tested were moderately-to-highly sensitive to viral infection. In addition, these cell lines also demonstrated the ability to support viral replication resulting in the exponential amplification of infectious virions. Conversely, five cell lines were resistant to viral infection. While the mechanism of resistance of cancer cells to viral infection remains largely unknown, our laboratory is actively exploring the genetic and molecular biologic factors thought to be involved.
These viruses have the potential to infect and selectively replicate within cancer cells—sparing normal cells(11). The specificity of oncolytic viruses in cancer therapy depends upon an interplay of the intrinsic properties of these viruses and cellular alterations of transformed cells. For example, the second-generation HSV mutant G207, containing mutations in genes required for viral replication, including ribonuclease reductase (RR), are dependent on a high level of homologous host-cell enzymes--characteristic of cancer cells--to complete its lifecycle(17;54). The specificity of oncolytic HSV for malignant cells—sparing normal hepatocytes—in the context of HCC and hepatic metastases has already been explored by our laboratory using an in vivo model of liver regeneration. Delman et al. investigated the ability of normal murine hepatocytes to permit viral replication of G207 following portal administration(55). Using histochemical staining for the viral marker gene lac Z and immunohistochemical and quantitative polymerase chain reaction-based detection of viral particles, the authors demonstrated no measurable viral presence or replication in normal, resting murine hepatocytes. Interestingly, this work did show that regenerating hepatocytes were capable of supporting viral replication during peak hepatocyte DNA synthesis following partial hepatectomy. The authors further demonstrated that this period of permissive viral replication correlated with upregulated hepatocyte ribonucleotide reductase activity—the viral homologue of which is deleted from G207 for attenuation. Viral administration immediately following resolution of liver regeneration, as early as seven days after hepatectomy, resulted in no measurable viral replication in the liver or any other organ.
While the maximum clinically achievable MOI in humans is not well-defined, it is generally thought that it approaches 1.0. More important, however, is the potential for in vivo amplification of the administered dose of this novel form of therapy—unlike all other standard therapies. As such, delivery of large initial doses is not necessary. Successful completion of the viral life cycle within cancer cells, in addition to causing cell death via lysis or apoptosis, results in the extracellular release of infectious viral progeny, and hence, an exponential amplification of the effective dose. Viral amplification is limited only to the extent that there are viable cancer cells present to support viral replication. Several human clinical trials investigating the use of oncolytic herpes viruses as anti-cancer agents have begun to answer this question. A phase I trial from our institution was the first to demonstrate that oncolytic herpes viruses can be safely injected into the bloodstream of patients with colorectal hepatic metastases. The maximum therapeutic dose was not reached but was determined to be greater than 1.3 x 107 pfu(56). In a phase I trial investigating the effectiveness of G207 in the treatment of recurrent gliomas, investigators delivered a maximum dose of 3 x 109 pfu directly into tumors(57;58).
Current management of hepatocellular carcinoma is inadequate and alternative treatment options are needed. Oncolytic herpes viruses have already been demonstrated to be effective in the treatment of colorectal liver metastases via portal and hepatic arterial delivery in animal models and human clinical trials are presently ongoing. This work provides a framework for future studies exploring the in vivo effectiveness of oncolytic HSV as potential therapy for patients with hepatocellular carcinoma.
Conclusions
This study demonstrates that an oncolytic herpes virus effectively infects, replicates within, and lyses the majority of all known human hepatocellular carcinoma cell lines. We suggest from this data that oncolytic HSV therapy may have a role in the treatment of HCC in the future. Additionally, this compilation of all commercially available HCC cell lines can be used to guide further in vitro and in vivo experiments for the treatment of hepatocellular carcinoma with oncolytic herpes viruses and other experimental treatment modalities.
Acknowledgments
The authors thank Medigene, Inc. for providing us with the G207 virus. Special thanks to Liza Marsh of the Department of Surgery at Memorial Sloan-Kettering Cancer Center for her assistance in preparing this manuscript.
Footnotes
Supported by grants R01CA75461 and R01CA72632 from the National Institutes of Health, and by MBC-99366 from the American Cancer Society
Presented in part at the meeting of the American Hepato-Pancreato-Biliary Association Congress April 14-17 2005, Fort Lauderdale, FL.
References
- 1.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. Tp53 and liver carcinogenesis. Human Mutation. 2003;21:201–16. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 2.Wang XW, Hussain SP, Huo TI, Wu CG, Forgues M, Hofseth LJ, et al. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology. 2002;181–182:43–47. doi: 10.1016/s0300-483x(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 3.Okuda K. Hepatocellular carcinoma. Journal of Hepatology. 2000;32:225–37. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Statistics Presentation 2004. 2004. Ref Type: Generic
- 5.Toda M, Rabkin SD, Martuza RL. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Human Gene Therapy. 1998;9:2177–85. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 6.Todo T, Rabkin SD, Chahlavi A, Martuza RL. Corticosteroid administration does not affect viral oncolytic activity, but inhibits antitumor immunity in replication-competent herpes simplex virus tumor therapy. Hum Gene Ther. 1999;10:2869–78. doi: 10.1089/10430349950016591. [DOI] [PubMed] [Google Scholar]
- 7.Chahlavi A, Todo T, Martuza RL, Rabkin SD. Replication-competent herpes simplex virus vector G207 and cisplatin combination therapy for head and neck squamous cell carcinoma. Neoplasia. 1999;1:162–69. doi: 10.1038/sj.neo.7900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coukos G, Makrigiannakis A, Montas S, Kaiser LR, Toyozumi T, Benjamin I, et al. Multi-attenuated herpes simplex virus-1 mutant G207 exerts cytotoxicity against epithelial ovarian cancer but not normal mesothelium and is suitable for intraperitoneal oncolytic therapy. Cancer Gene Ther. 2000;7:275–83. doi: 10.1038/sj.cgt.7700130. [DOI] [PubMed] [Google Scholar]
- 9.Oyama M, Ohigashi T, Hoshi M, Nakashima J, Tachibana M, Murai M, et al. Intravesical and intravenous therapy of human bladder cancer by the herpes vector G207. Hum Gene Ther. 2000;11:1683–93. doi: 10.1089/10430340050111331. [DOI] [PubMed] [Google Scholar]
- 10.Cozzi P, Burke PB, Bhargava A, Heston W, Huryk B, Scardino PT, et al. Oncolytic viral gene therapy for prostate cancer using two attenuated, replication competent, genetically-engineered herpes simplex viruses. Prostate. 2002;53:95–100. doi: 10.1002/pros.10138. [DOI] [PubMed] [Google Scholar]
- 11.Stanziale SF, Fong Y. Novel approaches to cancer therapy using oncolytic viruses. Current Molecular Medicine. 2003;3:61–71. doi: 10.2174/1566524033361663. [DOI] [PubMed] [Google Scholar]
- 12.Ebright MI, Zager JS, Malhotra S, Delman KA, Weigel TL, Rusch VW, et al. Replication-competent herpes virus NV1020 as direct treatment of pleural cancer in a rat model. Journal of Thoracic & Cardiovascular Surgery. 2002;124:123–29. doi: 10.1067/mtc.2002.122297. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JJ, Malhotra S, Wong RJ, Delman K, Zager J, St Louis M, et al. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Annals Of Surgery. 2001;233:819–26. doi: 10.1097/00000658-200106000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett JJ, Kooby DA, Delman K, McAuliffe P, Halterman MW, Federoff H, et al. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. Journal of Molecular Medicine. 2000;78:166–74. doi: 10.1007/s001090000092. [DOI] [PubMed] [Google Scholar]
- 15.Carew JF, Kooby DA, Halterman MW, Federoff HJ, Fong Y. Selective infection and cytolysis of human head and neck squamous cell carcinoma with sparing of normal mucosa by a cytotoxic herpes simplex virus type 1 (G207) Human Gene Therapy. 1999;10:1599–606. doi: 10.1089/10430349950017608. [DOI] [PubMed] [Google Scholar]
- 16.Cozzi PJ, Malhotra S, McAuliffe P, Kooby DA, Federoff HJ, Huryk B, et al. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and NV1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J. 2001;15:1306–8. doi: 10.1096/fj.00-0533fje. [DOI] [PubMed] [Google Scholar]
- 17.Yazaki T, Manz HJ, Rabkin SD, Martuza RL. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Research. 1995;55:4752–56. [PubMed] [Google Scholar]
- 18.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 19.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–66. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 20.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. Journal Of Clinical Investigation. 1993;91:2837–43. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll NM, Chiocca EA, Takahashi K, Tanabe KK. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Annals Of Surgery. 1996;224:323–29. doi: 10.1097/00000658-199609000-00008. discussion 329–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. Journal of Virology. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of Immunological Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 25.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. Journal of Immunological Methods. 1983;64:313–20. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 26.Weidmann E, Brieger J, Jahn B, Hoelzer D, Bergmann L, Mitrou PS. Lactate dehydrogenase-release assay: a reliable, nonradioactive technique for analysis of cytotoxic lymphocyte-mediated lytic activity against blasts from acute myelocytic leukemia. Annals of Hematology. 1995;70:153–58. doi: 10.1007/BF01682036. [DOI] [PubMed] [Google Scholar]
- 27.Park JG, Lee SK, Hong IG, Kim HS, Lim KH, Choe KJ, et al. MDR1 gene expression: its effect on drug resistance to doxorubicin in human hepatocellular carcinoma cell lines. J Natl Cancer Inst. 1994;86:700–705. doi: 10.1093/jnci/86.9.700. [DOI] [PubMed] [Google Scholar]
- 28.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. Journal of the National Cancer Institute. 1977;59:221–26. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 29.Kang MS, Lee HJ, Lee JH, Ku JL, Lee KP, Kelley MJ, et al. Mutation of p53 gene in hepatocellular carcinoma cell lines with HBX DNA. International Journal Of Cancer. 1996;67:898–902. doi: 10.1002/(SICI)1097-0215(19960917)67:6<898::AID-IJC22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Research. 1996;56:3831–36. [PubMed] [Google Scholar]
- 31.Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, et al. Characterization of cell lines established from human hepatocellular carcinoma. International Journal Of Cancer. 1995;62:276–82. doi: 10.1002/ijc.2910620308. [DOI] [PubMed] [Google Scholar]
- 32.Alexander JJ, Bey EM, Geddes EW, Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. South African Medical Journal. 1976;50:2124–28. [PubMed] [Google Scholar]
- 33.Gilligan A, Bushmeyer S, Knowles BB. Variation in EGF-induced EGF receptor downregulation in human hepatoma-derived cell lines expressing different amounts of EGF receptor. Experimental Cell Research. 1992;200:235–41. doi: 10.1016/0014-4827(92)90169-9. [DOI] [PubMed] [Google Scholar]
- 34.Macnab GM, Urbanowicz JM, Geddes EW, Kew MC. Hepatitis-B surface antigen and antibody in Bantu patients with primary hepatocellular cancer. Br J Cancer. 1976;33:544–48. doi: 10.1038/bjc.1976.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai HF, Kaneko S, Honda M, Shirota Y, Kobayashi K. alpha-fetoprotein-producing hepatoma cell lines share common expression profiles of genes in various categories demonstrated by cDNA microarray analysis. Hepatology. 2001;33:676–91. doi: 10.1053/jhep.2001.22500. [DOI] [PubMed] [Google Scholar]
- 36.Heffelfinger SC, Hawkins HH, Barrish J, Taylor L, Darlington GJ. SK HEP-1: a human cell line of endothelial origin. In Vitro Cellular & Developmental Biology. 1992;28A:136–42. doi: 10.1007/BF02631017. [DOI] [PubMed] [Google Scholar]
- 37.Gucev ZS, Oh Y, Kelley KM, Labarta JI, Vorwerk P, Rosenfeld RG. Evidence for insulin-like growth factor (IGF)-independent transcriptional regulation of IGF binding protein-3 by growth hormone in SKHEP-1 human hepatocarcinoma cells. Endocrinology. 1997;138:1464–70. doi: 10.1210/endo.138.4.5060. [DOI] [PubMed] [Google Scholar]
- 38.Turner BM, Turner VS. Secretion of alpha 1-antitrypsin by an established human hepatoma cell line and by human/mouse hybrids. Somatic Cell Genetics. 1980;6:1–14. doi: 10.1007/BF01538692. [DOI] [PubMed] [Google Scholar]
- 39.ATCC. American Type Culture Collection. ATCC . 2004. 2004. Ref Type: Electronic Citation
- 40.Sharkey FE, Fogh J. Metastasis of human tumors in athymic nude mice. International Journal Of Cancer. 1979;24:733–38. doi: 10.1002/ijc.2910240605. [DOI] [PubMed] [Google Scholar]
- 41.KCLB. Korean Cell Line Bank. KCLB . 2005. 2004. Ref Type: Electronic Citation
- 42.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. International Journal Of Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 43.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. European Journal Of Cancer. 2001;37 (Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 44.El Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. New England Journal Of Medicine. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 45.Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institutes, and US Cancer Working Group. United States Cancer Statistics: 2000 Incidence. 50–77. 2003. Ref Type: Generic
- 46.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Annals Of Surgery. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamazoe R, Hirooka Y, Ohtani S, Katoh T, Kaibara N. Intraoperative microwave tissue coagulation as treatment for patients with nonresectable hepatocellular carcinoma. Cancer. 1995;75:794–800. doi: 10.1002/1097-0142(19950201)75:3<794::aid-cncr2820750308>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Fong Y. A promising technique for liver cancer? Cancer Journal From Scientific American. 1999;5:339–40. [PubMed] [Google Scholar]
- 49.Giorgio A, Ferraioli G. Radiofrequency thermal ablation versus percutaneous ethanol injection for small hepatocellular carcinoma. Radiology. 2004;230:886–87. doi: 10.1148/radiol.2303031235. [DOI] [PubMed] [Google Scholar]
- 50.Zuber-Jerger I, Geissler M, Spangenberg HC, Mohr L, Weizsacker F, Blum HE. Local ablation of malignant lesions of the liver - potential applications and limitations of the different methods. Zeitschrift fur Gastroenterologie. 2004;42:31–38. doi: 10.1055/s-2004-812687. [DOI] [PubMed] [Google Scholar]
- 51.Segawa T, Tsuchiya R, Furui J, Izawa K, Tsunoda T, Kanematsu T. Operative results in 143 patients with hepatocellular carcinoma. World J Surg. 1993;17:663–67. doi: 10.1007/BF01659138. [DOI] [PubMed] [Google Scholar]
- 52.Lau WY, Arnold M, Guo SK, Li AK. Microwave tissue coagulator in liver resection for cirrhotic patients. Australian & New Zealand Journal of Surgery. 1992;62:576–81. doi: 10.1111/j.1445-2197.1992.tb07053.x. [DOI] [PubMed] [Google Scholar]
- 53.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–40. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 54.Kramm CM, Chase M, Herrlinger U, Jacobs A, Pechan PA, Rainov NG, et al. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Human Gene Therapy. 1997;8:2057–68. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 55.Delman KA, Zager JS, Bhargava A, Petrowsky H, Malhotra S, Ebright MI, et al. Effect of murine liver cell proliferation on herpes viral behavior: implications for oncolytic viral therapy. Hepatology. 2004;39:1525–32. doi: 10.1002/hep.20198. [DOI] [PubMed] [Google Scholar]
- 56.Fong Y, Kemeny N, Jarnagin W, Stanziale S, Guilfoyle B, Gusani N, et al. Phase 1 study of a replication-competent herpes simplex oncolytic virus for treatment of hepatic colorectal metastases. Proc Am Soc Clin Oncol. 2002;21:8a. [Google Scholar]
- 57.Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther. 2004;11:643–64. doi: 10.1038/sj.cgt.7700733. [DOI] [PubMed] [Google Scholar]
- 58.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]


