Abstract
The purification of reaction mixtures is a slow process in organic synthesis, especially during the production of large numbers of analogs and compound libraries. Phase-tag methods such as solid-phase synthesis and fluorous synthesis, provide efficient ways of addressing the separation issue. Fluorous synthesis employs functionalized perfluoroalkyl groups attached to substrates or reagents. The separation of the resulting fluorous molecules can be achieved using strong and selective fluorous liquid-liquid extraction, fluorous silica gel-based solid-phase extraction or high-performance liquid chromatography. Fluorous technology is a novel solution-phase method, which has the advantages of fast reaction times in homogeneous environments, being readily adaptable to literature conditions, having easy intermediate analysis, and having flexibility in reaction scale and scope. In principle, any synthetic methods that use a solid-support could be conducted in solution-phase by replacing the polymer linker with a corresponding fluorous tag. This review summarizes the progress of fluorous tags in solution-phase synthesis of small molecules, peptides and oligosaccharides.
Abbreviations: μW Microwave, Boc tert-Butoxycarbonyl, BTF Benzotrifluoride, Cbz Benzyloxycarbonyl, DIC Diisopropylcarbodiimide, DMAP 4-Dimethylaminopyridine, FC-72 Perfluorohexane, F-LLE Fluorous liquid-liquid extraction, Fmoc 9-Fluorenylmethoxycarbonyl, F-SPE Fluorous solid-phase extraction, HPLC High-performance liquid chromatography, PMB 4-Methoxybenzyl, Rf Perfluoroalkyl group
Introduction
Phase tagging is a useful strategy for the purification of reaction mixtures [1•], and has been successfully employed in solid-phase peptide synthesis. Further application of solid-phase synthesis for small molecules, however, has not yet fulfilled the high expectations of chemists. Small-molecule synthesis has a much broader scope than peptide synthesis, and the solid-phase approach for small molecules is limited by unfavorable heterogeneous reaction kinetics, longer development times and difficulties in analyzing resin-bound intermediates. With a paradigm shift away from the synthesis of libraries containing millions of compounds toward much smaller libraries, solution-phase methods, such as polymer-assisted synthesis [2] and fluorous synthesis [3••,4•,5•], have gained popularity in recent years.
Fluorous synthesis employs functionalized perfluoroalkyl groups (Rf) as phase tags [6••]. This technology has inherited the characteristics of solution-phase synthesis, ie, fast reaction times in a homogeneous environment, adaptability to literature conditions, easy analysis of intermediates and flexibility in reaction scale and scope. Fluorous tags are usually attached to the parent molecules via a (CH2)m segment to insulate the reactive site from the electron-withdrawing fluorine atoms (a fluorous alkyl chain CnF2n+1-(CH2)m can be abbreviated as RfnHm). In fluorous synthesis, the functional group of the fluorous molecules controls the reaction, while the fluorous tag dominates the separation. Fluorous tags can be categorized as ‘heavy’ or ‘light’. Heavy tags contain multiple Rf chains and have a fluorine content greater than 60% of the molecular weight, while light tags contain only one or two Rf chains with significantly fewer fluorine atoms. Fluorous separation is based on strong and selective fluorine-fluorine interactions between the fluorous molecules and the fluorous separation media [7•]. Liquid-liquid extraction (LLE) with fluorous and organic solvents has been used for the separation of heavy fluorous molecules, while silica gel-based fluorous solid-phase extraction (F-SPE) and high-performance liquid chromatography (HPLC) have been used for the separation of light fluorous molecules.
A broad technology platform of fluorous chemistry has been available since the early 1990s. The original fluorous tagging strategy for catalyst recycling [8] has been extended to substrate tagging and reagent/scavenger tagging. Fluorous molecules have been applied to many areas of organic synthesis [9], including biphasic catalyses, thermomorphic catalyses, triphasic reactions, mixture syntheses [10•], multicomponent reactions, microwave (μW) reactions, ion reactions and solid-phase liquid reactions, supercritical CO2 reactions. This review discusses a substrate-tagging strategy for the parallel synthesis of small molecules, peptides and oligosaccharides.
Fluorous tags are the derivatives of protecting groups such as tert-butoxycarbonyl (Boc), 4-methoxybenzyl (PMB), benzyloxycarbonyl (Cbz) and silyl groups, or Wang, Rink and Marshall linkers. Some representative fluorous tags for ‘catch and release’ of substrates are listed in Table 1.
Table 1.
Representative catch and release fluorous tags.
| Abbreviation | Tag | Tagged substrate/reagent | Reference |
|---|---|---|---|
| F-Silyl |
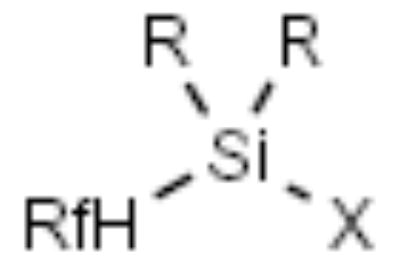
|
Alcohols | [11,12] |
| F-PMB (Wang-type) |
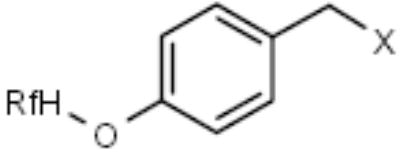
|
Alcohols | [13] |
| F-Boc |
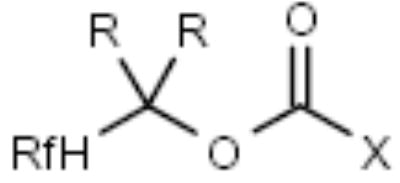
|
Amines | [14] |
| F-Cbz |
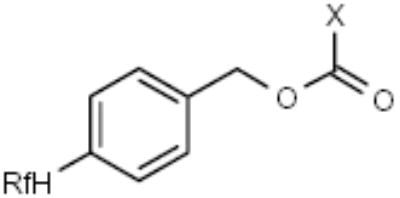
|
Amines | [15,16] |
| F-THP |
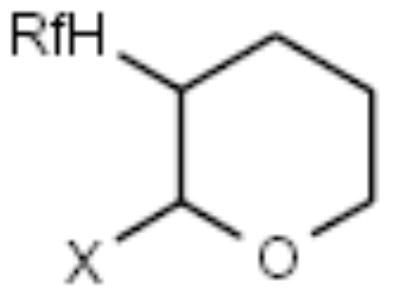
|
Alcohols | [17] |
| F-Alkoxyethene |
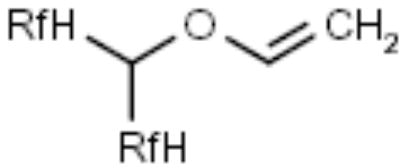
|
Alcohols, amines | [18] |
| F-tert-Butanol |
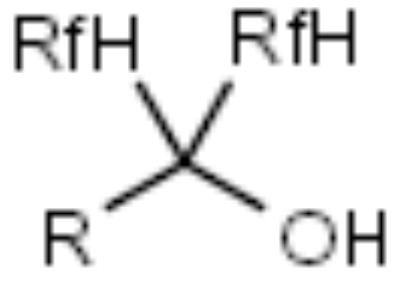
|
Carboxylic acids | [19] |
| F-Alcohol |
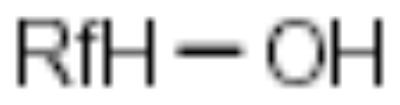
|
Carboxylic (amino) acids | [20,21] |
| F-Thiol |
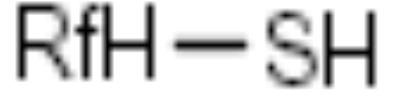
|
Activated halides | [22] |
| F-Sulfonyl |
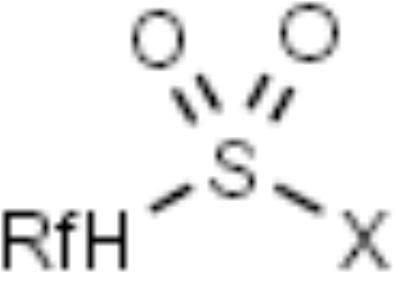
|
Phenols | [23] |
| FluoMar (Marshall-type) |
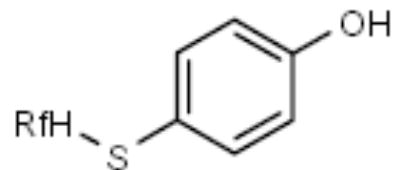
|
Carboxylic acids | [24] |
| F-Diol |
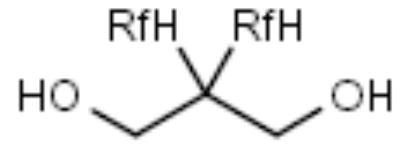
|
Ketones, aldehydes | [25] |
| F-Benzophenone imine |
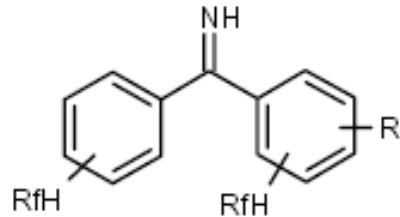
|
Halides | [26] |
| F-Benzaldehyde |
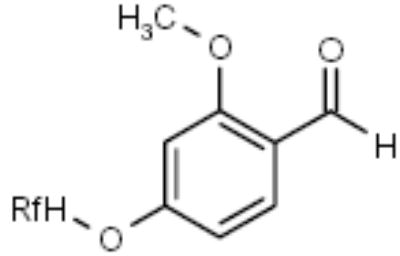
|
Amines | [27] |
X Leaving group.
Fluorous silyl
Studer and Curran reported the synthesis of isoxazolines 4 (Scheme 1A) and isoxazoles 7 (Scheme 1B) by cycloaddition of nitrile oxides 1 with fluorous silyl-protected allyl and propargyl alcohols (compounds 2 and 5, respectively; Scheme 1) [11]. An excess amount of nitrile oxides 1 were used to drive the cycloaddition reaction to completion.
Scheme 1.
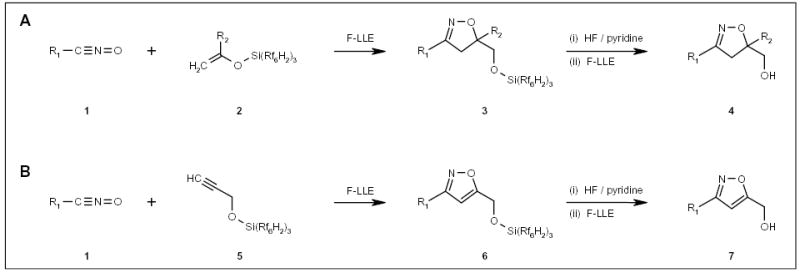
The application of a fluorous silyl tag for isoxazole and isoxazoline synthesis.
Unreacted nitrile oxides were isolated by fluorous liquid-liquid extraction (F-LLE) with perfluorohexane (FC-72)/benzene/water. Following desilylation with hydrogen fluoride and pyridine, final products 4 and 7 were purified by a second liquid extraction with FC-72, dichloromethane and aqueous ammonium chloride.
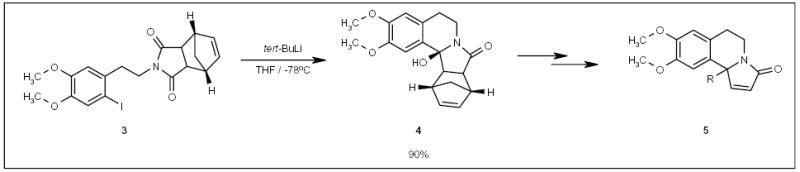
The combination of fluorous and solid-phase technologies for small-molecule synthesis has been reported by Wipf and co-workers [101]. Resin-bound intermediates 8 (Scheme 2), prepared by several steps of solid-phase synthesis, were attached to a fluorous silyl group (tert-butyl-phenyl-((2-perfluorooctyl)ethyl) silyl (BPFOS)), and the resulting fluorous molecule 9, along with untagged byproducts accumulated from previous steps, were cleaved from the polymer support to provide the fluorous tagged product 10 (Scheme 2). Fluorous product 10 was isolated by F-SPE and subjected to further reactions to afford oxazole and thioazole analogs of curacin (compounds 11; Scheme 2).
Scheme 2.
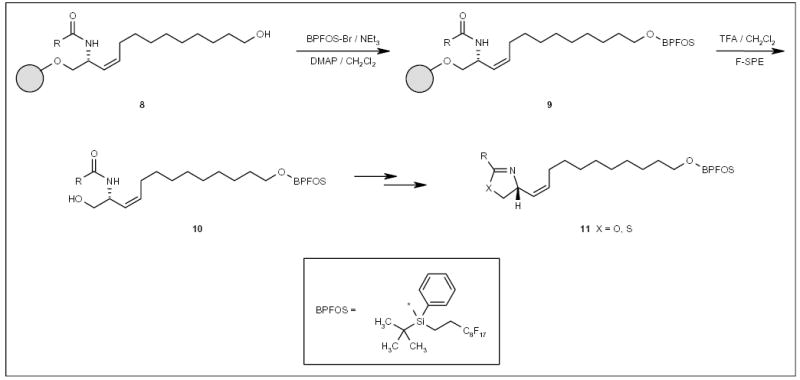
The application of a fluorous silyl tag for oxazole and thioazole synthesis.
The Wipf and Curran research groups also conducted a multicomponent Biginelli reaction using fluorous urea 12 (Scheme 3) as the limiting agent, while other components such as β-ketone ester 13 and aldehyde 14 were used in excess with a mixture of tetrahydrofuran (THF) and benzotrifluoride (BTF) [28]. The condensed fluorous dihydropyrimidine intermediates (not shown) were isolated by FC-84 extraction, and the cleaved fluorous tag was removed from the reaction mixture by a second FC-84 extraction to produce pyrimidine-2-one derivative 15 (Scheme 3).
Scheme 3.
The application of a fluorous silyl tag in a Biginelli reaction.
Fluorous tert-butoxycarbonyl
Fluorous Boc (F-Boc) has been employed as an amino protecting group in the parallel synthesis of a small library of isonipecotic acid derivatives 20 (Scheme 4) [14]. Fluorous intermediate 17 was coupled with a number of different amines to provide amides 18. Following deprotection with trifluoroacetic acid (TFA), the resulting piperidine amide compounds 19 were further reacted with an array of halide compounds, resulting in a 96-compound library. All of the reaction mixtures containing fluorous components were purified by F-SPE.
Scheme 4.
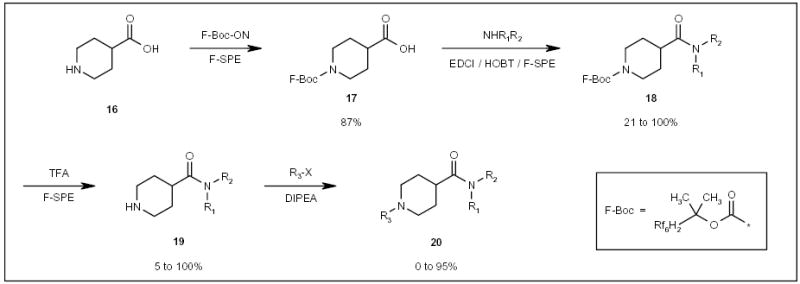
The application of an F-Boc protecting group for the synthesis of isonipecotic acid derivatives.
EDCI 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide, HOBT 1-hydroxybenzotriazole, DIPEA N,N-diisopropylethylamine.
The synthetic efficiency of an Ugi reaction/de-Boc/cyclization method, originally developed by the Hulme research group, has been improved by the application of fluorous and •W technologies [29]. In the synthesis of quinoxalinone 26 and benzimidazole 29 (Scheme 5A and 5B, respectively) using F-Boc protected aniline 22, the time for the Ugi reaction was reduced from 36 to 48 h to less than 20 min under μW irradiation. The removal of excess aldehydes and unreacted acids from the reaction mixture was accomplished using F-SPE instead of double-scavenging with polymer-supported tosylhydrazide and N,N-diisopro-pylethylamine (DIPEA). The deprotection of the F-Boc group with TFA and purification of the final product were also benefited by a faster μW reaction and easy F-SPE separation.
Scheme 5.
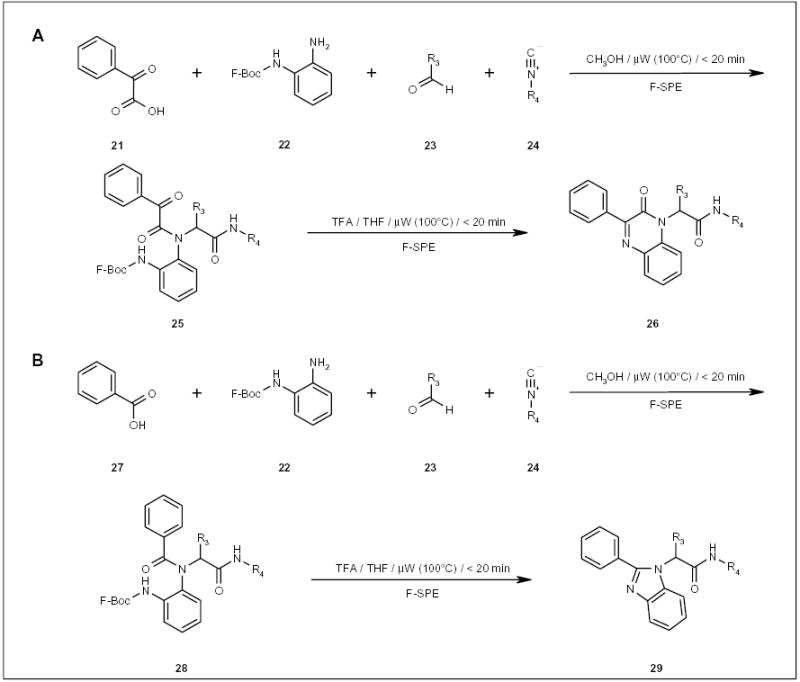
The μW-assisted fluorous synthesis of quinoxalinones and benzimidazoles.
Fluorous benzyloxycarbonyl
Schwinn and Bannwarth employed fluorous Cbz (F-Cbz)-protected anilines in the synthesis of quinazoline-2,4-diones 33 (Scheme 6) [30]. Amidation of F-Cbz-protected acids 31, followed by cyclative deprotection of furans 32 in an FC-72 layer, led to the formation of products 33, which were extracted into an ethyl acetate layer and purified by F-LLE.
Scheme 6.
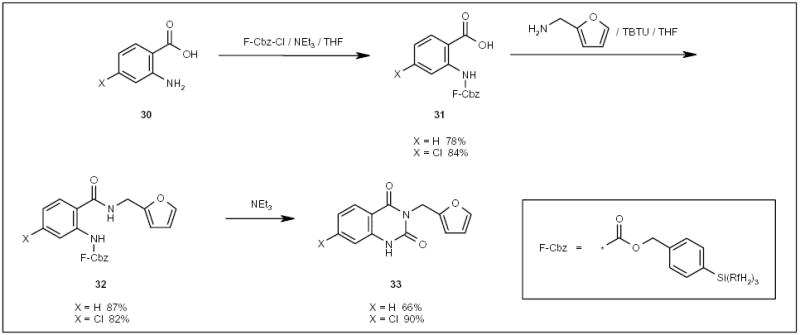
The application of an F-Cbz protecting group in the synthesis of quinazoline-2,4-diones.
TBTU O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate.
This chemistry has since been improved by the same research group through absorption of the fluorous molecules onto fluorous silica gel via strong fluorine-fluorine interactions to eliminate the requirement for fluorous solvents in both the reaction and separation steps [31].
Fluorous alcohols
Wipf and Methot accomplished the synthesis of dihydropyridazinone 40 (Scheme 7) using fluorous alcohol-protected carboxylic acid 36 (Scheme 7) [20]. The δ-keto ester 38 was treated with hydrazine to form a dihydro-pyridazinone ring (Scheme 7). The fluorous tag was cleaved during the cyclization, and the resulting product 39 was separated from the reaction mixture by F-LLE with FC-72 and methanol.
Scheme 7.
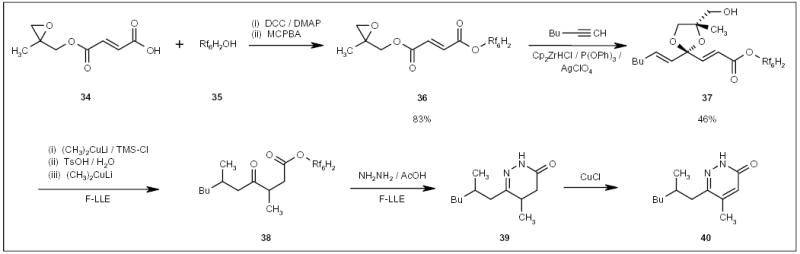
The fluorous alcohol-protected synthesis of a dihydropyridazinone.
DCC Dicyclohexylcarbodiimide, DMAP dimethylaminopyridine, MCPBA 3-chloroperoxybenzoic acid, TMS trimethylsilyl.
Fluorous amino acids have been used in the parallel synthesis of a hydantoin/thiohydantoin library 45 (Scheme 8) [21]. Two fluorous-tagged amino acids 41 (Scheme 8) having different R1 groups underwent reductive amination with six different aldehydes 42. Each of the 12 intermediates 43 were reacted with ten aryl isocyanates/aryl isothiocyanates. The resulting ureas/thioureas 44 were spontaneously cyclized to displace the fluorous tag and form heterocyclic products 45.
Scheme 8.
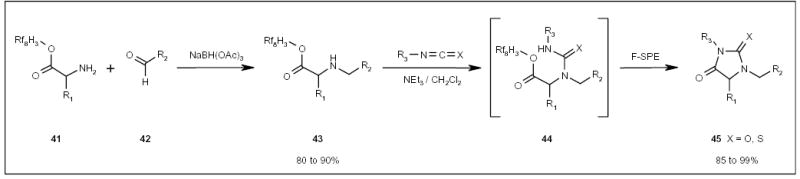
The fluorous synthesis of a substituted hydantoin/thiohydantoin library.
Fluorous amino acids have also been employed in the synthesis of an N-alkylated dihydropteridinone library 54 (Scheme 9) [32]. 4,6-Dichloro-5-nitropyrimidine (46) was first displaced with fluorous amino acids 47, and then displaced with secondary amines 49 (Scheme 9). The reduction of the nitro group of nitropyrimidines 50 was conducted by hydrogenation using Pd/C as a catalyst, and this was followed by a cyclization reaction of aminopyrimi-dines 51, promoted by μW irradiation. The N-alkylation reaction of fused heterocyclic compounds 52 with benzyl halides 53 (Scheme 9) gave mono products 54 in high selectivity, using 1.0 equivalent of a base and 1.0 equivalent of benzyl halides 53 in the present of lithium bromide.
Scheme 9.
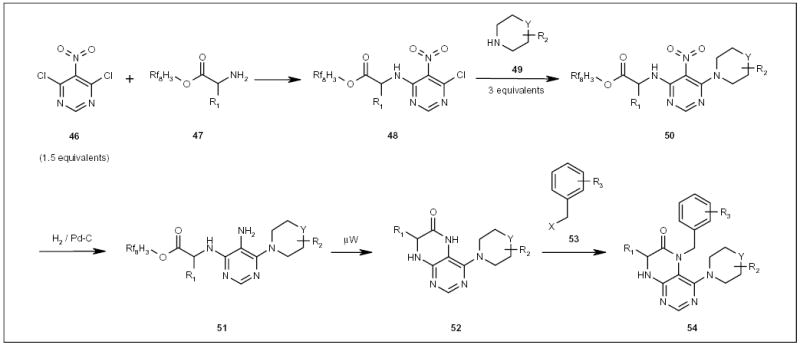
The fluorous synthesis of dihydropteridinone derivatives.
Fluorous amino esters 57 (Scheme 10) have been used in the synthesis of proline analogs via three-component reactions [Zhang W, Lu Y, Chen C-T, unpublished results]. 1,3-Dipolar cycloaddition of pyrrolidine diones 55, benzaldehydes 56 and amino ester 57, gave fused heterocycle 58 as a single diastereomer, which was purified by F-SPE. Adducts 58 have been used as key intermediates in the construction of novel tricyclic compounds, such as compounds 59 and 60 (Scheme 10).
Scheme 10.
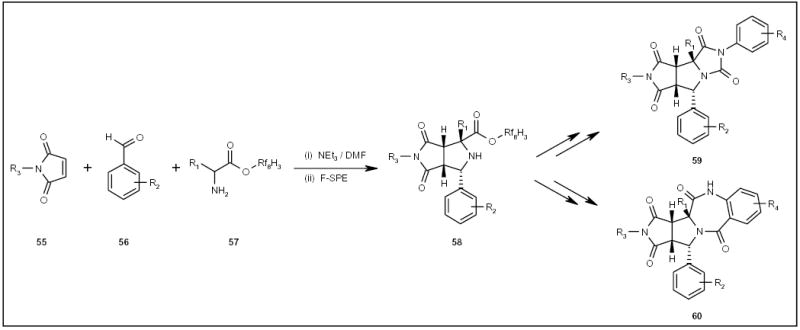
The fluorous synthesis of novel heterocyclic systems.
Fluorous diols
The utility of fluorous diols as carbonyl group protecting agents has been demonstrated by Read and Zhang in the synthesis of 4-phenyl-pyridine derivative 68 (Scheme 11) [Read R, Zhang C, personal communications]. One of the carbonyl groups of 1,4-benzodialdehyde (61) was protected with fluorous diol 62. The resulting protected compound 63 underwent condensation, cycloaddition and oxidation reactions, and was finally deprotected with hydrochloric acid to afford substituted pyridine 68.
Scheme 11.
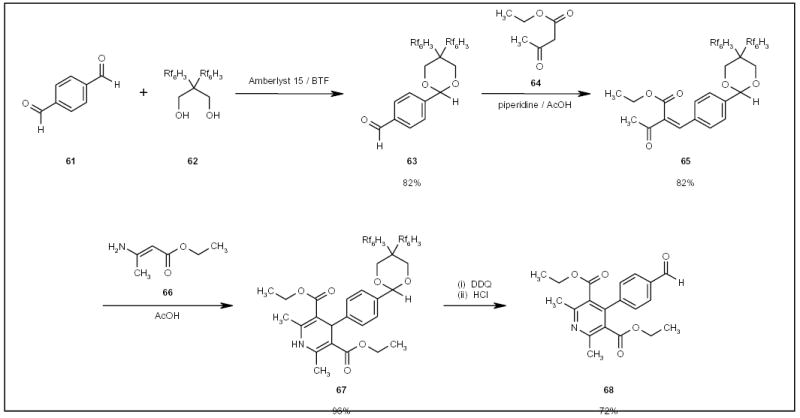
The fluorous synthesis of a substituted pyridine.
DDQ Dichlorodicyanoquinone.
Fluorous thiols
A fluorous thiol group has been used as a nucleophilic tag to anchor 2,4-dichloropyrimidine 69 (Scheme 12) [22]. The tagged substrate 71 was further displaced with 3-(trifluoromethyl)pyrazole (72) to give pyrazolylpyrimidine 73 (Scheme 12). The thiol tag was then activated by oxidation to sulfone 74 and displaced by a set of nucleophiles (Nu) to afford disubstituted pyrimidines 75 (Scheme 12).
Scheme 12.
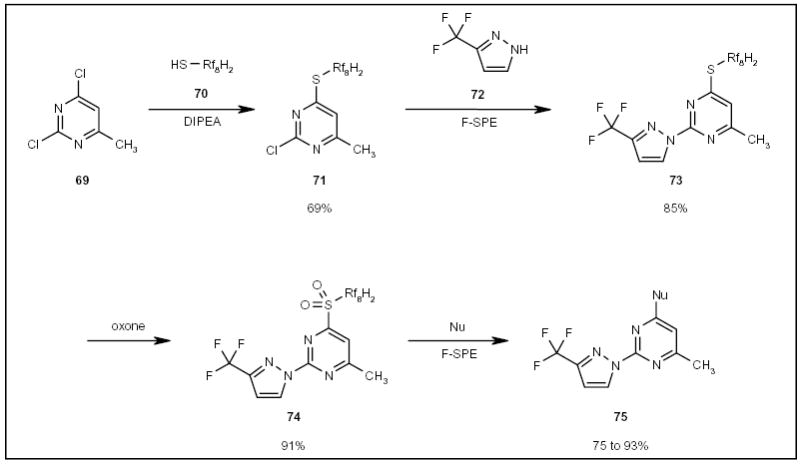
The application of a fluorous thiol tag for the synthesis of a disubstituted pyrimidine analog.
FluoMarTM
FluoMarTM (77; Scheme 13) is a fluorous version of the Marshall resin, and has been used as a catch and release tag in the parallel synthesis of amides 81 (Scheme 13) [24]. FluoMarTM was attached to carboxylic acids 76 under general coupling conditions with diisopropylcarbodiimide (DIC) and dimethylaminopyridine (DMAP). The tagged compound 78 (Scheme 13) underwent Boc deprotection and acylation to give amide 80. The fluorous tag was then displaced with a set of amines to give diamides 81.
Scheme 13.
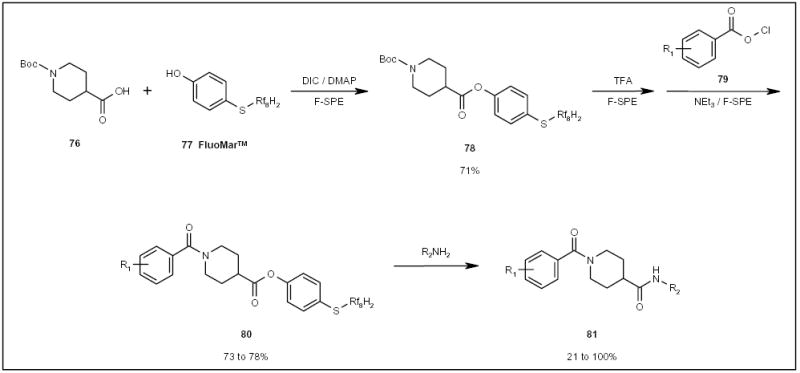
The application of a FluoMarTM tag in the parallel synthesis of diamides.
Fluorous benzophenone imines
Herr and co-workers employed fluorous benzophenone imine 82 (Scheme 14) to tag aryl halides 83 or triflates and converted them to corresponding amines 85 by hydrolysis of N-aryl benzophenone imines 84 (Scheme 14) [26]. Fluorous benzophenone was recovered by F-SPE for the regeneration of 82.
Scheme 14.
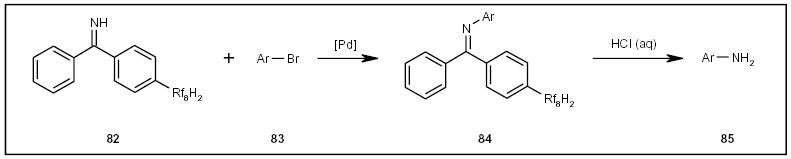
The fluorous synthesis of anilines.
Fluorous sulfonyls
The perfluorooctylsulfonyl group has been used to tag phenols, with the resulting F-sulfonates having similar reactivity to triflates, which have been used in cross-coupling reactions to form aryl C-C [23], C-S [33] and C-H [34] bonds. Such cross-coupling reactions have been accelerated by μW irradiation.
The fluorous sulfonyl tag has been employed in multistep syntheses, for example, the fluorous benzaldehyde 86 (Scheme 15A) underwent a reductive amination to form compound 88, which was then reacted with an isocyanate 89 (Scheme 15B) to form substituted hydantoin 90 or with a benzoyl chloride 93 to form amide 94. F-SPE-purified
Scheme 15.
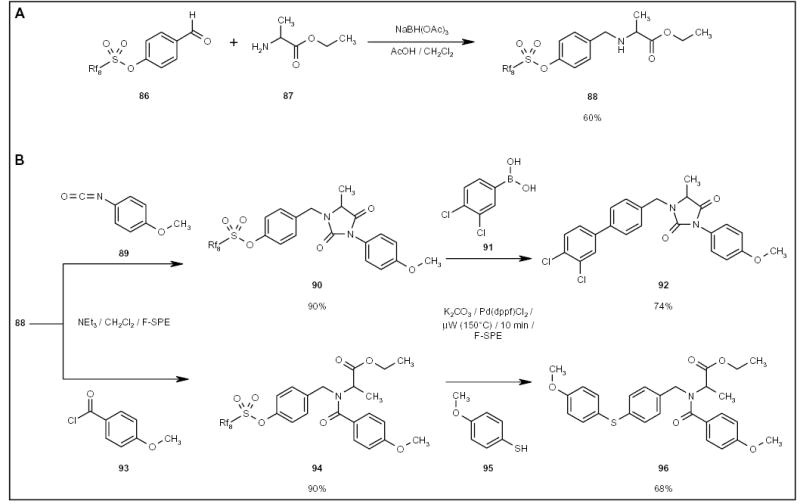
The application of an F-sulfonyl tag for multistep synthesis.
F-sulfonates 90 and 94 were used for palladium-catalyzed cross-couplings to form the corresponding biaryl 92 [23] and aryl sulfide 96 [33] compounds, respectively. In the multistep synthesis, the fluorous sulfonyl tag facilitated the intermediate purification, and also served as a hydroxyl-protecting group during the early steps of the reaction and activated the hydroxyl group for the coupling reaction at the last step.
μW and fluorous technologies have been employed in the solution-phase parallel synthesis of a substituted 3-aminoimidazo[1,2-a]pyridine and 3-aminoimidazo[1,2-a]pyrazine library (compounds 101; Scheme 16) [35]. Multicomponent reactions of perfluorooctylsulfonyl-tagged benzaldehydes 97 with isonitriles 98 and 2-amino-pyridines/2-aminopyrazines 99 produced compounds 100 (Scheme 16). The condensation products were subjected to Pd-catalyzed cross-coupling reactions with boronic acids or thiols to form imidazopyridines or imidazopyrazines 101 (Scheme 16).
Scheme 16.
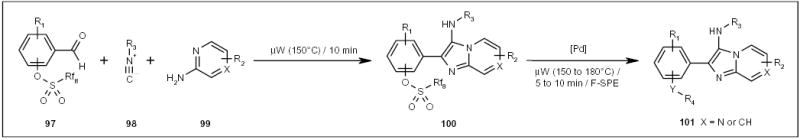
The application of an F-sulfonyl tag for multicomponent and subsequential cross-coupling reactions.
Fluorous benzaldehyde
Ladlow and co-workers recently developed an acid-labile fluorous benzaldehyde protecting group 102 to facilitate the parallel synthesis of sulfonamides 107 (Scheme 17) [27]. All intermediates and the final products were purified by F-SPE.
Scheme 17.
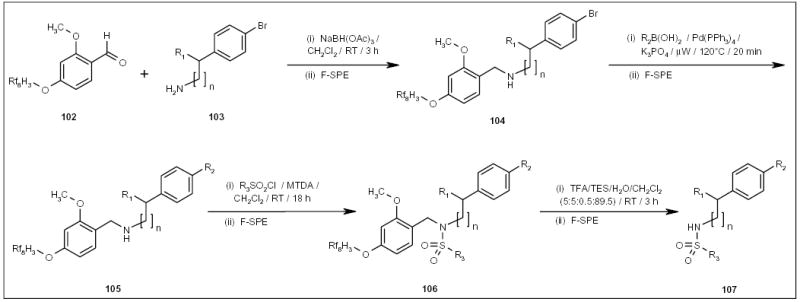
The fluorous synthesis of sulfonamides.
MTDA Methyl trimethylsilyl dimethylketene acetal, TES triethylsilyl.
Peptide synthesis
The application of fluorous tags to solid-phase peptide synthesis was first reported by van Boom and co-workers [36]. In a 9-fluorenylmethoxycarbonyl (Fmoc)-based amino acid synthesis, the unreacted free amines were capped with the acetyl group after each condensation step (Scheme 18) [36]. After the desired number of iterations, the deprotection of the final Fmoc group was followed by tagging with an F-Cbz moiety. A mixture containing the desired F-tagged product and acetyl-capped byproducts were separated by HPLC with a fluorous column. Using this method, the van Boom research group was able to purify peptide oligomers containing seven to 22 amino acids. Recently, Overkleeft and co-workers introduced a base-labile fluorous methylsulfonylethoxycarbonyl (F-Msc) moiety as an alternative protecting group for Fmoc-based peptide synthesis [37].
Scheme 18.
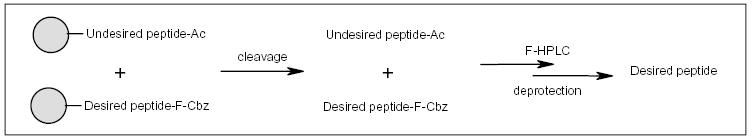
The application of F-Cbz for peptide synthesis.
Inazu and co-workers applied a Rink-type protecting group with a fluorous support containing six perfluorooctyl groups (Hfb), ie, compound 108 (Scheme 19), to peptide synthesis [38]. An excess amount of an amino acid derivative was used in each coupling step. The unreacted reagent and coupling agents were removed by extraction with methanol from the FC-72 layer. The product 109 was extracted into an FC-72 layer and was deprotected and purified to give pure tripeptide 110. The Mizuno research group recently reported a new PMB-type protecting group also using a fluorous Hfa support for peptide synthesis [39].
Scheme 19.
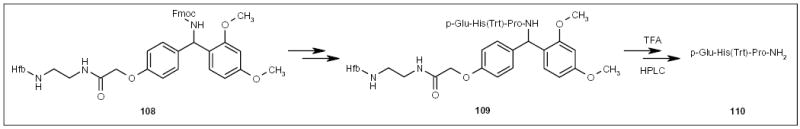
The application of a fluorous Rink-type tag for peptide synthesis.
Trt Triphenylmethyl.
Recently, Kumar an co-workers developed a trivalent iodonium compound 112 and used it as a capping reagent to remove deletion sequences up to 21 residues in length (Scheme 20) [40]. The fluorous byproducts were separated by reverse-phase chromatography or by precipitation following the addition of water to the reaction mixture.
Scheme 20.
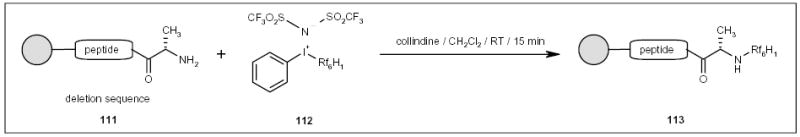
The application of a fluorous capping reagent for peptide synthesis.
Oligosaccharide synthesis
A fluorous tagging strategy has also been applied to the synthesis of oligosaccharides. Curran and co-workers employed fluorous benzyl (F-Bn) to protect hydroxyl groups in the synthesis of disaccharide 118 (Scheme 21) [41]. Tribenzyl tagged d-glucal 114 was coupled with excess diacetone galactose 115, under standard reaction conditions and in BTF, to give pure fluorous disaccharide 116 (Scheme 21) after triphasic extraction. Fluorous compound 116 was then debenzylated by catalytic hydrogenation with H2 and palladium(II) hydroxide in FC-72. After the triphasic extraction, product 117 was acylated in the organic phase to give disaccharide 118 (Scheme 21) in a methanol layer.
Scheme 21.
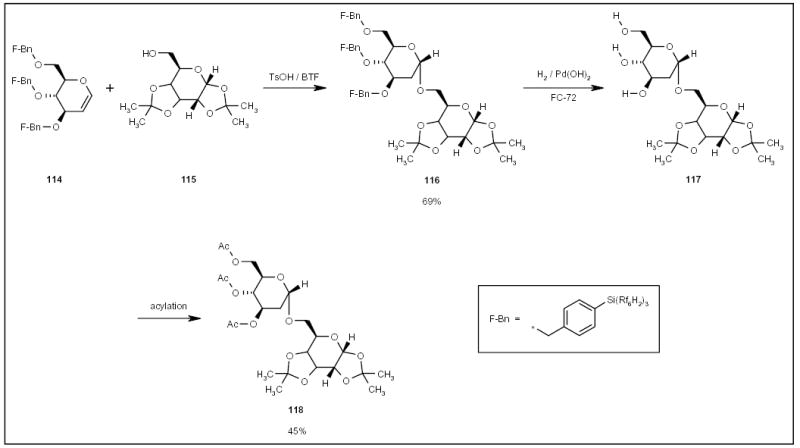
The application of an F-Bn tag for disaccharide synthesis.
Inazu and co-workers employed a fluorous bisperfluorooctyl acid tag (Bfp; Scheme 22) to protect the hydroxyl groups of a mannose derivative [42,43]. The triphenylmethyl (Trt) group of mannose derivative 119 was selectively removed by treatment with 10-camphorsulfonic acid (CSA). The deprotected hydroxyl group was then coupled to galactose derivative 120 to give fluorous disaccharide 121 (Scheme 22). Deprotection of both the acetyl and Bfp groups, followed by FC-72/methanol extraction gave disaccharide 122 in the methanol layer. The Bfp protecting group was recovered from the FC-72 layer as a methyl ester.
Scheme 22.
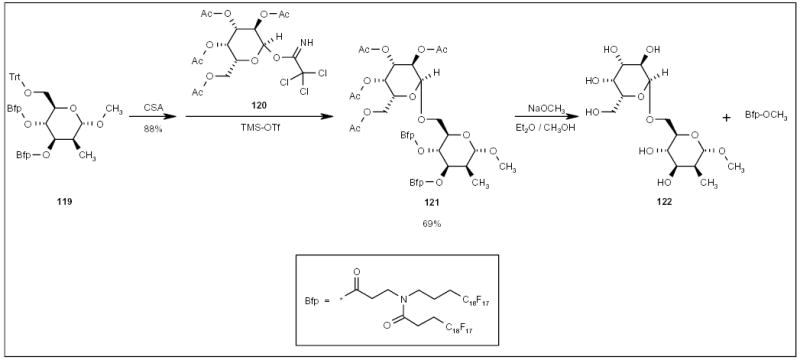
The application of a fluorous Bfp tag for disaccharide synthesis.
TMS-OTf Trimethylsilyl triflate.
Jing and Huang developed fluorous thiol 123 (Scheme 22) and used it to displace the bromo group of a tetraacetyl-α-glucosyl bromide 124 [44]. After removal of the acetate, the thiolglucoside 125 was subjected to benzoylation and glycosylation to give disaccharide 127 (Scheme 23) in good yield and high purity following F-SPE. The fluorous thiol 123 could be recycled.
Scheme 23.
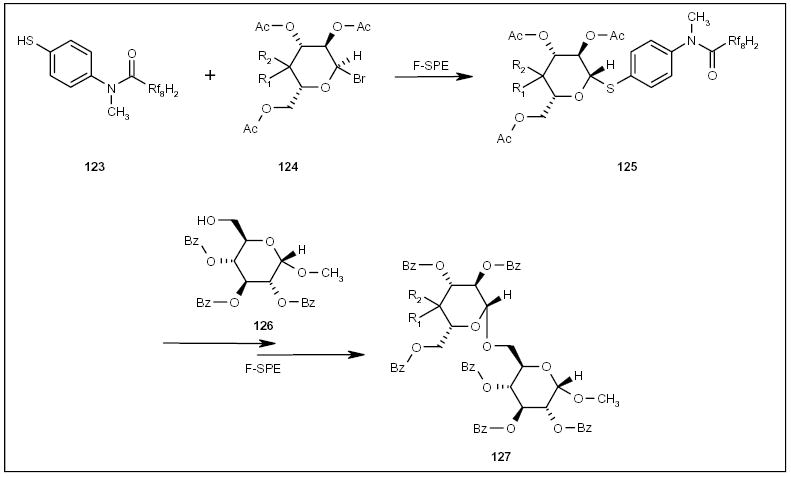
The application of a fluorous thiol tag to disaccharide synthesis.
Instead of tagging the substrates, Seeberger and co-workers employed fluorous silyl protecting groups to cap the hydroxyl group of the undesired sequences [45]. Since the desired oligomeric species were non-fluorous, they could be separated from the fluorous byproducts by HPLC and no detagging step was required.
Manzoni recently reported the use of fluorous silyl reagent 129 (Scheme 24) to protect the anomeric position of sugar acceptors in saccharide synthesis. The tagged substrates 130 were purified by F-SPE [46].
Scheme 24.
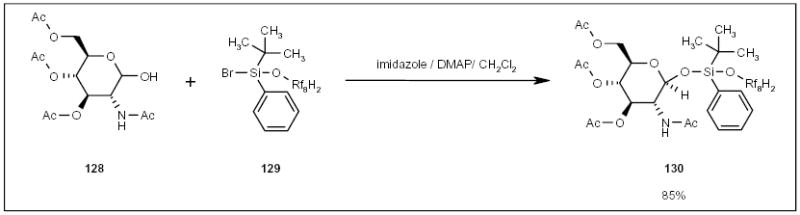
The application of a fluorous silyl tag for saccharide synthesis.
Conclusion
Fluorous synthesis has favorable solution-phase reaction kinetics and overcomes some of the disadvantages of solid-phase synthesis. This new high-throughput technology also has the characteristics of easy reaction monitoring and a broad synthetic scope. The linker strategy developed for solid-phase synthesis can be applied to solution-phase fluorous synthesis by simply replacing the polymer support with a fluorous tag. With the increasing availability of fluorous tagging agents, organic chemists should find additional applications of fluorous technologies in the preparation of small molecules, peptides and oligosaccharides.
References
•• of outstanding interest
• of special interest
- 1.Yoshida J-I, Itami K. Tag strategy for separation and recovery. Chem Rev. 2002;102(10):3693–3716. doi: 10.1021/cr0103524. An excellent review on phase tagging, including solid and fluorous supports. [DOI] [PubMed] [Google Scholar]
- 2.Ley SV, Baxendale IR, Bream RN, Jackson PS, Leach AG, Longbottom DA, Nessi M, Scott JS, Storer RI, Taylor SJ. Multi-step organic synthesis using solid-supported reagents and scavengers: A new paradigm in chemical library generation. J Chem Soc Perkin Trans. 2000;1(23):3815–4195. [Google Scholar]
- 3.••.Gladysz JA, Horvath IT, Curran DP (EDS): The Handbook of Fluorous Chemistry. Wiley-VCH, Weinheim, Germany (2004).A comprehensive and updated reference book on fluorous synthesis and separations.
- 4.Zhang W. Fluorous synthesis of heterocyclic systems. Chem Rev. 2004;104(5):2531–2556. doi: 10.1021/cr030600r. A review on the synthesis of heterocyclic compounds using fluorous tags, reagents, scavengers and other fluorous technologies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W. Fluorous technologies for solution-phase high-throughput organic synthesis. Tetrahedron. 2003;59(25):4475–4489. A review on ‘light fluorous synthesis’ using fluorous silica gel-based separation. [Google Scholar]
- 6.••.Curran DP. Strategy-level separations in organic synthesis: From planning to practice. Angew Chem Int Ed. 1998;37(9):1175–1196. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1174::AID-ANIE1174>3.0.CO;2-P. An original review on fluorous tagging strategy for organic synthesis. [DOI] [PubMed] [Google Scholar]
- 7.Curran DP. Fluorous reverse phase silica gel. A new tool for preparative separations in synthetic organic and organofluorine chemistry. Synlett. 2001;(9):1488–1496. A review on the fluorous silica gel-based SPE and HPLC separations. [Google Scholar]
- 8.Horva’th I. Fluorous biphase chemistry. Acc Chem Res. 1998;31 (10):641–650. [Google Scholar]
- 9.Curran DP. Fluorous methods for synthesis and separation of organic molecules. Pure Appl Chem. 2000;72(9):1649–1653. [Google Scholar]
- 10.Zhang W: Fluorous mixture synthesis (FMS) of enantiomers, diastereomers and compound libraries Arkivoc (2004) (i):101–109.Summary of the application of fluorous tags for the mixture synthesis of individually pure isomers and compound libraries. [PMC free article] [PubMed]
- 11.Studer A, Curran DP. A strategic alternative to solid phase synthesis: Preparation of a small isoxazoline library by ‘fluorous synthesis’. Tetrahedron. 1997;53(19):6681–6696. [Google Scholar]
- 12.Röver S, Wipf P. Synthesis and applications of fluorous silyl protecting groups with improved acid stability. Tetrahedron Lett. 1999;40(31):5667–5670. [Google Scholar]
- 13.Curran DP, Furukawa T. Simultaneous preparation of four analogues of discodermolide by fluorous mixture synthesis. Org Lett. 2002;4(13):2233–2235. doi: 10.1021/ol026084t. [DOI] [PubMed] [Google Scholar]
- 14.Luo ZY, Williams J, Read RW, Curran DP. Fluorous Boc ((F)Boc) carbamates: New amine protecting groups for use in fluorous synthesis. J Org Chem. 2001;66(12):4261–4266. doi: 10.1021/jo010111w. [DOI] [PubMed] [Google Scholar]
- 15.Filippov DV, van Zoelen DJ, Oldfield SP, van der Marel GA, Overkleeft HS, Drijfhoutb JW, van Boom JH. Use of benzyloxycarbonyl (Z)-based fluorophilic tagging reagents in the purification of synthetic peptides. Tetrahedron Lett. 2002;43(43):7809–7812. [Google Scholar]
- 16.Curran DP, Amatore M, Guthrie D, Campbell M, Go E, Luo Z. Synthesis and reactions of fluorous carbobenzyloxy (fCbz) derivatives of □-amino acids. J Org Chem. 2003;68(12):4643–4647. doi: 10.1021/jo0344283. [DOI] [PubMed] [Google Scholar]
- 17.Wipf P, Reeves JT. Synthesis and applications of a fluorous THP protective group. Tetrahedron Lett. 1999;40(31):4649–4652. [Google Scholar]
- 18.Wipf P, Reeves JT. Synthesis and applications of a highly fluorous alkoxy ethyl ether protective group. Tetrahedron Lett. 1999;40 (28):5139–5142. [Google Scholar]
- 19.Pardo J, Cobas A, Guitian E, Castedo L. Fluorinated analogues of tert-butyl alcohol as novel protecting groups for use in fluorous synthesis. Org Lett. 2001;3(16):3711–3714. doi: 10.1021/ol0166505. [DOI] [PubMed] [Google Scholar]
- 20.Wipf P, Methot J-L. Silver(I)-catalyzed addition of zirconocenes to epoxy esters: A new entry to 1,4-dicarbonyl compounds and pyridazinones. Org Lett. 1999;1(8):1253–1255. [Google Scholar]
- 21.Zhang W, Lu Y. Fluorous synthesis of hydantoins and thiohydantoins. Org Lett. 2003;5(14):2555–2558. doi: 10.1021/ol034854a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W. Fluorous synthesis of disubstituted pyrimidines. Org Lett. 2003;5(7):1011–1013. doi: 10.1021/ol027469e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Chen CH, Lu Y, Nagashima T. A highly efficient microwave-assisted Suzuki coupling reaction of aryl perfluorooctylsulfonates with boronic acids. Org Lett. 2004;6 (9):1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CH, Zhang W. FluoMar, a fluorous version of the Marshall resin for solution-phase library synthesis. Org Lett. 2003;5(7):1015–1017. doi: 10.1021/ol0274864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read RW, Zhang C. Synthesis of fluorous acetal derivatives of aldehydes and ketones. Tetrahedron Lett. 2003;44(37):7045–7047. [Google Scholar]
- 26.Cioffi CL, Berlin ML, Herr RJ: Convenient palladium-catalyzed preparation of primary anilines using a fluorous benzophenone imine reagent Synlett (2004) (5):841–845.
- 27.Villard A-L, Warrington BH, Ladlow M. A fluorous-tagged, acid-labile protecting group for the synthesis of carboxamides and sulfonamides. J Combinatorial Chem. 2004;6(4):611–622. doi: 10.1021/cc0499338. [DOI] [PubMed] [Google Scholar]
- 28.Studer A, Jeger P, Wipf P, Curran DP. Fluorous synthesis: Fluorous protocols for the Ugi and Biginelli multicomponent condensations. J Org Chem. 1997;62(9):2917–2924. doi: 10.1021/jo970095w. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Tempest P. Highly efficient microwave-assisted fluorous Ugi and post-condensation reactions for benzimidazoles and quinoxalinones. Tetrahedron Lett. 2004;45(36):6757–6760. [Google Scholar]
- 30.Schwinn D, Bannwarth W. Perfluoro-tagged benzyloxycarbonyl protecting group and its application in fluorous biphasic systems. Helv Chim Acta. 2002;85(1):255–264. [Google Scholar]
- 31.Schwinn D, Glatz H, Bannwarth W. Multistep parallel synthesis of quinazoline-2,4-diones by a fluorous biphasic concept without perfluorinated solvents. Helv Chim Acta. 2003;86(1):188–195. [Google Scholar]
- 32.Nagashima T, Zhang W: Solution-phase parallel synthesis of an N-alkylated dihydropteridinone library from fluorous amino acids J Comb Chem6(6):942–949. [DOI] [PubMed]
- 33.Zhang W, Lu Y, Chen CH. Combination of microwave reactions with fluorous separations in the palladium-catalyzed synthesis of aryl sulfides. Mol Diversity. 2003;7(2–4):199–202. doi: 10.1023/b:modi.0000006825.12186.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Nagashima T, Lu Y, Chen CH-T. A traceless perfluorooctylsulfonyl tag for deoxygenation of phenols under microwave irradiation. Tetrahedron Lett. 2004;45(24):4611–4613. [Google Scholar]
- 35.Lu Y, Zhang W:Microwave-assisted synthesis of a 3-aminoimidazo[1,2-a]-pyridine/pyrazine library by fluorous multicomponent reactions and subsequent cross-coupling reactions (2004):Manuscript submitted. [DOI] [PMC free article] [PubMed]
- 36.Filippov DV, van Zoelen DJ, Oldfield SP, van der Marel GA, Overkleeft HS, Drijfhout JW, van Boom JH. Use of benzyloxycarbonyl (Z)-based fluorophilic tagging reagents in the purification of synthetic peptides. Tetrahedron Lett. 2002;43(43):7809–7812. [Google Scholar]
- 37.de Visser PC, van Helden M, Filtppov DV, van der Marel GA, Drijfhout JW, van Boom JH, Noort D, Overkleeft HS. A novel, base-labile fluorous amine protecting group: Synthesis and use as a tag in the purification of synthetic peptides. Tetrahedron Lett. 2003;44 (50):9013–9016. [Google Scholar]
- 38.Mizuno M, Goto K, Miura T, Hosaka D, Inazu T: A novel peptide synthesis using fluorous chemistry.Chem Commun (2003) (8):972–973. [DOI] [PubMed]
- 39.Mizuno M, Goto K, Miura T, Matsuura T, Inazu T. Peptide synthesis on fluorous support. Tetrahedron Lett. 2004;45(17):3425–3428. [Google Scholar]
- 40.Montanari V, Kumar K. Just add water: A new fluorous capping reagent for facile purification of peptides synthesis on the solid phase. J Am Chem Soc. 2004;126(31):9528–9529. doi: 10.1021/ja0479033. [DOI] [PubMed] [Google Scholar]
- 41.Curran DP, Ferritto R, Hua Y. Preparation of a fluorous benzyl protecting group and its use in fluorous synthesis approach to a disaccharide. Tetrahedron Lett. 1998;39(28):4937–4940. [Google Scholar]
- 42.Miura T, Goto K, Hosaka D, Inazu T. Oligosacchride synthesis on a fluorous support. Angew Chem Int Ed. 2003;42(18):2047–2051. doi: 10.1002/anie.200250531. [DOI] [PubMed] [Google Scholar]
- 43.Miura T, Goto K, Waragai H, Matsumoto H, Hirose Y, Ohmae M, Ishida H-K, Satoh A, Inazu T. Rapid oligosaccharide synthesis using a fluorous protecting group. J Org Chem. 2004;69(16):5348–5353. doi: 10.1021/jo049425k. [DOI] [PubMed] [Google Scholar]
- 44.Jing Y, Huang X. Fluorous thiols in oligosaccharide synthesis. Tetrahedron Lett. 2004;45(24):4615–4618. [Google Scholar]
- 45.Palmacci ER, Hewitt MC, Seeberger PH. ‘Cap-tag’ - novel methods for the rapid purification of oligosaccharide prepared by automated solid-phase synthesis. Angew Chem Int Ed. 2001;40(23):4433–4437. doi: 10.1002/1521-3773(20011203)40:23<4433::aid-anie4433>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Mazoni L: Rapid synthesis of oligosaccharides using an anomeric fluorous silyl protecting group.Chem Commun (2003) (23):2930–2931. [DOI] [PubMed]
References to patent literature
- 101.U niversity of Pittsburgh (Wipf P, Reeves J, Röver S): Fluorous tagging compounds and methods of use thereof. US-06673539 (2004).


