Abstract
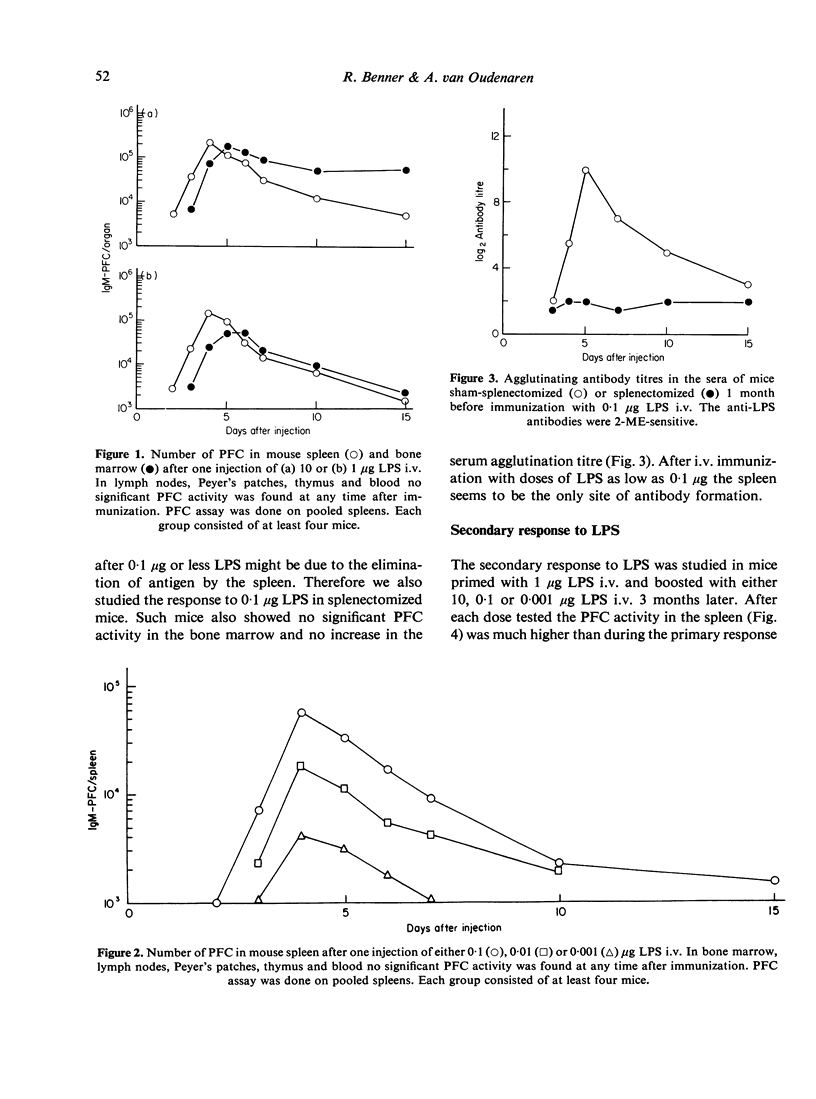
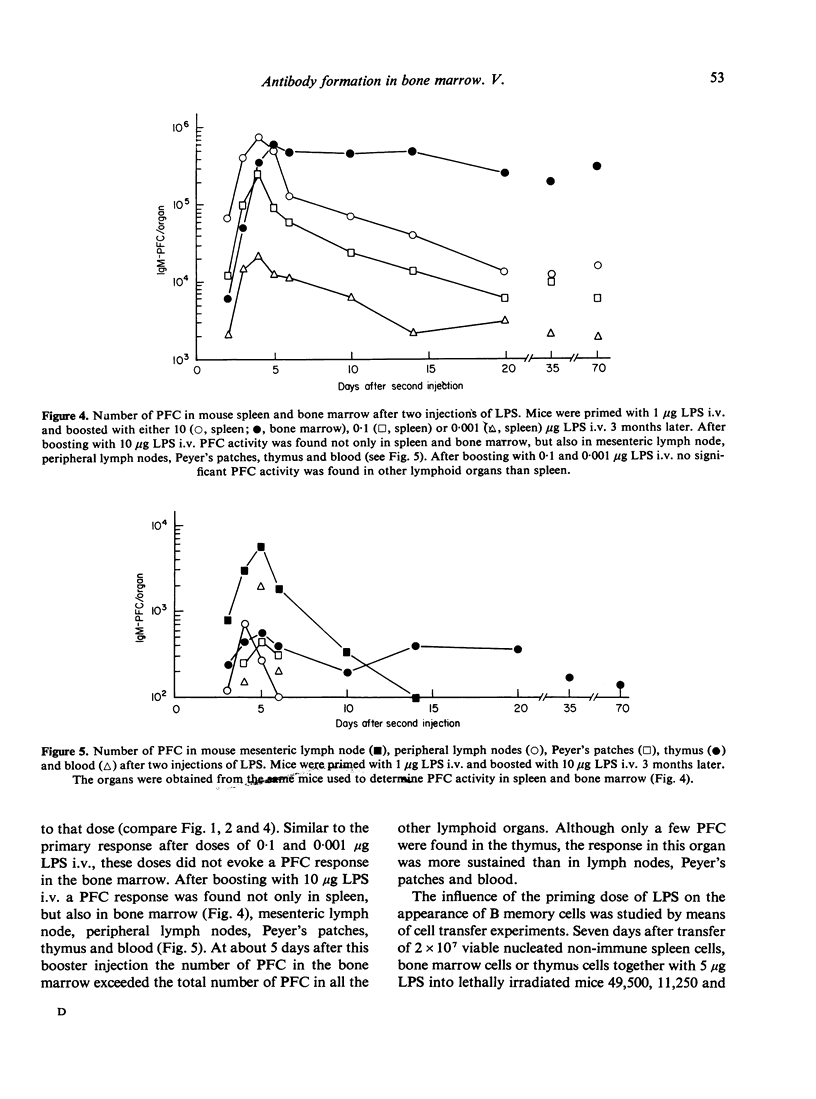
The occurrence of plaque-forming cells (PFC) in mouse bone marrow was studied during primary and secondary responses to the thymus-independent antigen Escherichia coli lipopolysaccharide (LPS). Anti-LPS responses were induced by various doses of LPS. During the primary response, doses of 1 and 10 mug LPS intravenously (i.v.) were found to evoke a distinct PFC response in both spleen and bone marrow. The spleen contained the majority of PFC until about 5 days after immunization. During the course of the reaction the number of PFC in the bone marrow rose to a level which equalled or surpassed the level in the spleen. LPS doses of 0-001, 0-01 and 0-1 mug i.v. only induced a PFC response in the spleen. Apparently there is a minimal threshold dose of LPS of about 1 mug for PFC to appear in the bone marrow. The secondary response was studied in mice primed with 1 mug LPS i.v. and boosted with either 0-001, 0-1 or 10 mug LPS i.v. 3 months later. After each dose tested the PFC activity in the spleen was several times higher than during the primary response. As was observed in the primary response doses of 0-001 and 0-1 mug LPS i.v. did not evoke a PFC response in the bone marrow. After boosting with 10 mug of LPS i.v. a significant PFC response was found in spleen, bone marrow, thymus, lymph nodes, Peyer's patches and blood. From about 5 days after the booster injection the number of PFC in the bone marrow exceeded the total number found in all other lymphoid organs. The results are discussed in relation to the bone marrow PFC response to the thymus-dependent antigen sheep red blood cells. To this antigen a clear PFC response in the bone marrow is found only during the secondary response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaskov J. G., Halliday W. J. Requirement for lymphocyte-macrophage interaction in the response of mouse spleen cultures to pneumococcal polysaccharide. Cell Immunol. 1971 Aug;2(4):335–340. doi: 10.1016/0008-8749(71)90068-2. [DOI] [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B., SEBESTYEN M. M. Effect of bacterial endotoxins on the reticuloendothelial system. Fed Proc. 1957 Sep;16(3):860–867. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., Van der Meulen G. M. B memory cells in the thymus: resistance to corticosteroid treatment in vivo and to anti theta treatment in vitro. Cell Immunol. 1974 Oct;14(1):151–154. doi: 10.1016/0008-8749(74)90180-4. [DOI] [PubMed] [Google Scholar]
- Benner R., Meima F., Van der Meulen G. M., van Ewijk W. Antibody formation in mouse bone marrow. III. Effects of route of priming and antigen dose. Immunology. 1974 Nov;27(5):747–760. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., van der Meulen G. M. Antibody formation in mouse bone marrow. II. Evidence for a memory-dependent phenomenon. Cell Immunol. 1974 Jul;13(1):95–106. doi: 10.1016/0008-8749(74)90230-5. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A. Antibody formation in mouse bone marrow. IV. The influence of splenectomy on the bone marrow plaque-forming cell response to sheep red blood cells. Cell Immunol. 1975 Oct;19(2):167–182. doi: 10.1016/0008-8749(75)90201-4. [DOI] [PubMed] [Google Scholar]
- Bona C., Robineaux R., Anteunis A., Heuclin C., Astesano A. Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide. Immunology. 1973 May;24(5):831–840. [PMC free article] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Peters L. C. Requirement for continuous antigenic stimulation in the development and differentiation of antibody-forming cells: effect of antigen dose. Immunology. 1971 May;20(5):707–718. [PMC free article] [PubMed] [Google Scholar]
- Jehn U. W., Karlin L. Independent action of thymus and bone marrow cells during the secondary response of direct plaque-forming cells. J Immunol. 1971 Apr;106(4):946–950. [PubMed] [Google Scholar]
- Landy M., Sanderson R. P., Jackson A. L. Humoral and cellular aspects of the immune response to the somatic antigen of Salmonella enteritidis. J Exp Med. 1965 Sep 1;122(3):483–504. doi: 10.1084/jem.122.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Petrov R. V., Mikhajlova A. A., Stepanenko R. N., Zakharova L. A. Cell interactions in the immune response: effect of humoral factor released from bone marrow cells on the quantity of mature antibody producers in culture of immune lymph node cells. Cell Immunol. 1975 Jun;17(2):342–350. doi: 10.1016/s0008-8749(75)80038-4. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]


