Abstract
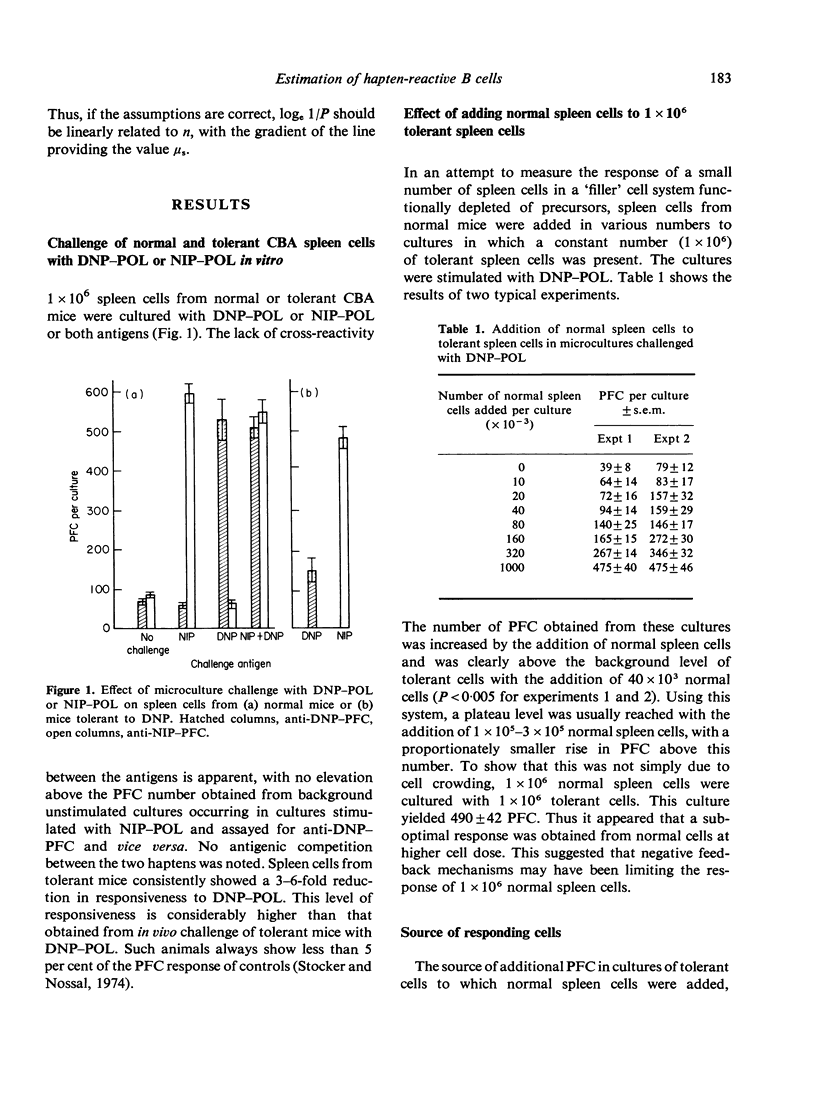
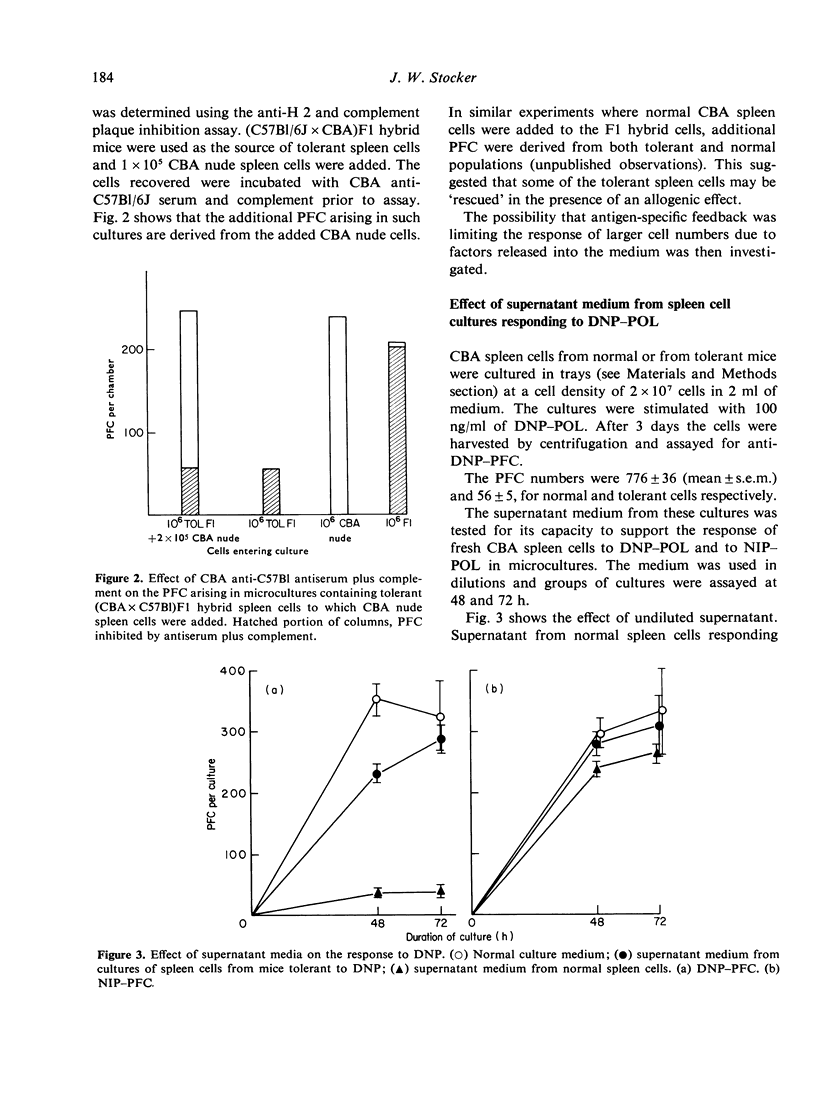
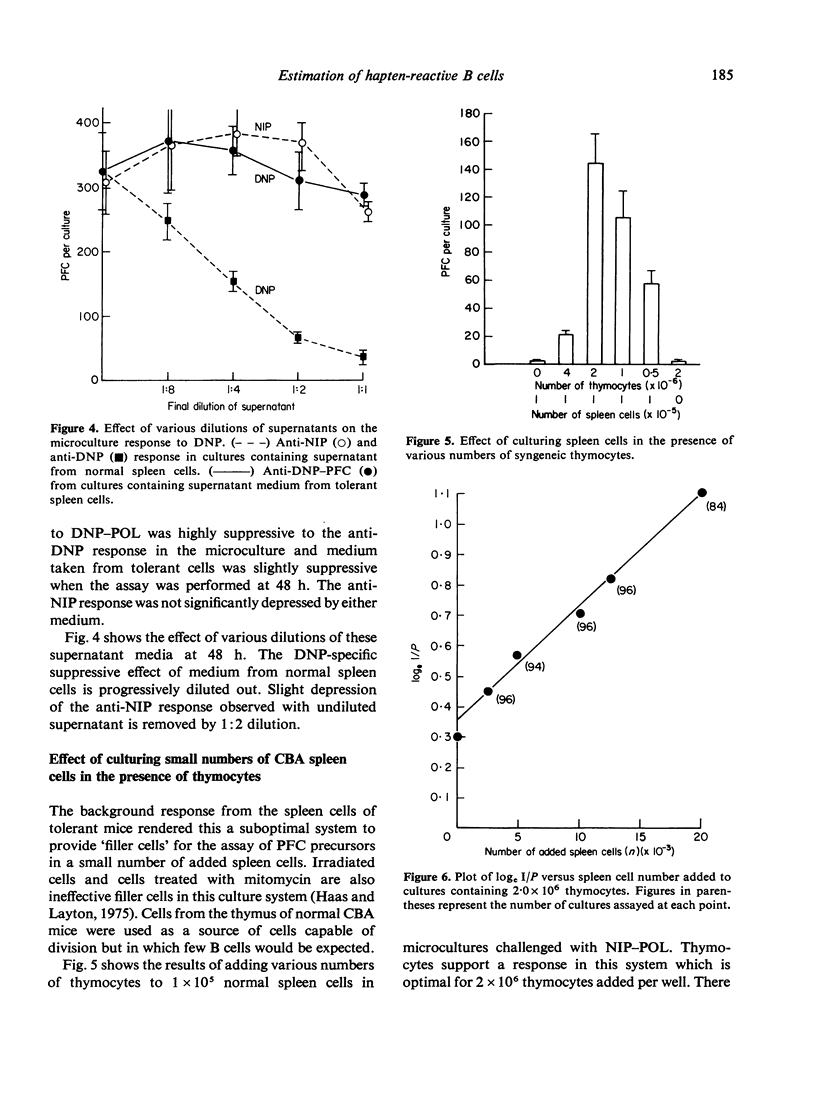
The immune response of mouse spleen cells to hapten-conjugated polymer of flagellin (DNP-POL, NIP-POL) was studied using a microculture system. When increasing numbers of spleen cells were added to a 'filler' cell system, negative feedback effects became apparent and resulted in the generation of progressively lower numbers of plaque-forming cells (PFC) per input cell. This feedback inhibition was shown to be antigen-specific and mediated by factors released into the culture medium. The effect precludes calculation of the frequency of PFC precursors in cultures containing spleen cells alone and complicates the analysis of tolerance using in vitro assay systems. The addition of small numbers of spleen cells to a constant number of thymocytes provided a system in which Poisson analysis could be used to determine the frequency of PFC precursors capable of being activated by hapten-POL conjugates. This system was used to estimate the frequency of anti-NIP-PFC precursors in CBA spleen cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstone A., Mitchison N. A., Pitt-Rivers R. Chemical and serological studies with an iodine-containing synthetic immunological determinant 4-hydroxy-3-iodo-5-nitrophenylacetic acid (NIP) and related compounds. Immunology. 1966 May;10(5):465–479. [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Salk J. Effects of IgM on the in vivo and in vitro immune response. Proc Soc Exp Biol Med. 1973 Sep;143(4):889–893. doi: 10.3181/00379727-143-37435. [DOI] [PubMed] [Google Scholar]
- Diener E., Feldmann M. Antibody-mediated suppression of the immune response in vitro. II. A new approach to the phenomenon of immunological tolerance. J Exp Med. 1970 Jul 1;132(1):31–43. doi: 10.1084/jem.132.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N. PREPARATION OF PURIFIED ANTI-2,4-DINITROPHENYL ANTIBODIES. Methods Med Res. 1964;10:94–102. [PubMed] [Google Scholar]
- Haas W., Layton J. E. Separation of antigen-specific lymphocytes. I. Enrichment of antigen-binding cells. J Exp Med. 1975 May 1;141(5):1004–1014. doi: 10.1084/jem.141.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W. Separation of antigen-specific lymphocytes. II. Enrichment of hapten-specific antibody-forming cell precursors. J Exp Med. 1975 May 1;141(5):1015–1029. doi: 10.1084/jem.141.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchet R., Feldmann M. Tolerance induction to a hapten protein conjugate in vivo: are suppressor T cells involved. Eur J Immunol. 1974 Nov;4(11):768–771. doi: 10.1002/eji.1830041112. [DOI] [PubMed] [Google Scholar]
- Lefkovits I. Induction of antibody-forming cell clones in microcultures. Eur J Immunol. 1972 Aug;2(4):360–366. doi: 10.1002/eji.1830020412. [DOI] [PubMed] [Google Scholar]
- Marbrook J., Haskill J. S. The in vitro response to sheep erythrocytes by mouse spleen cells: segregation of distinct events leading to antibody formation. Cell Immunol. 1974 Jul;13(1):12–21. doi: 10.1016/0008-8749(74)90222-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W. Immune responses in vitro. II. Suppression of the immune response in vitro by specific antibody. J Exp Med. 1969 Aug 1;130(2):365–379. doi: 10.1084/jem.130.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintáns J., Lefkovits I. Precursor cells specific to sheep red cells in nude mice. Estimation of frequency in the microculture system. Eur J Immunol. 1973 Jul;3(7):392–397. doi: 10.1002/eji.1830030704. [DOI] [PubMed] [Google Scholar]
- Radcliffe G. N., Axelrad M. A. Immunological memory in vitro. J Exp Med. 1971 Apr 1;133(4):846–856. doi: 10.1084/jem.133.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W. Regulation of the immune response by IgM antibody: a paradoxical suppression of the in vitro primary immune response to sheep erythrocytes by passive IgM. Aust J Exp Biol Med Sci. 1973 Jun;51(3):333–346. doi: 10.1038/icb.1973.32. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Nossal G. J. Induction of B lymphocyte tolerance by an oligovalent antigen. I. Influence of the read-out system. Cell Immunol. 1975 Mar;16(1):162–170. doi: 10.1016/0008-8749(75)90195-1. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Osmond D. G., Nossal G. J. Differentiation of lymphocytes in the mouse bone marrow. III. The adoptive response of bone marrow cells to a thymus cell-independent antigen. Immunology. 1974 Nov;27(5):795–806. [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]


