Abstract
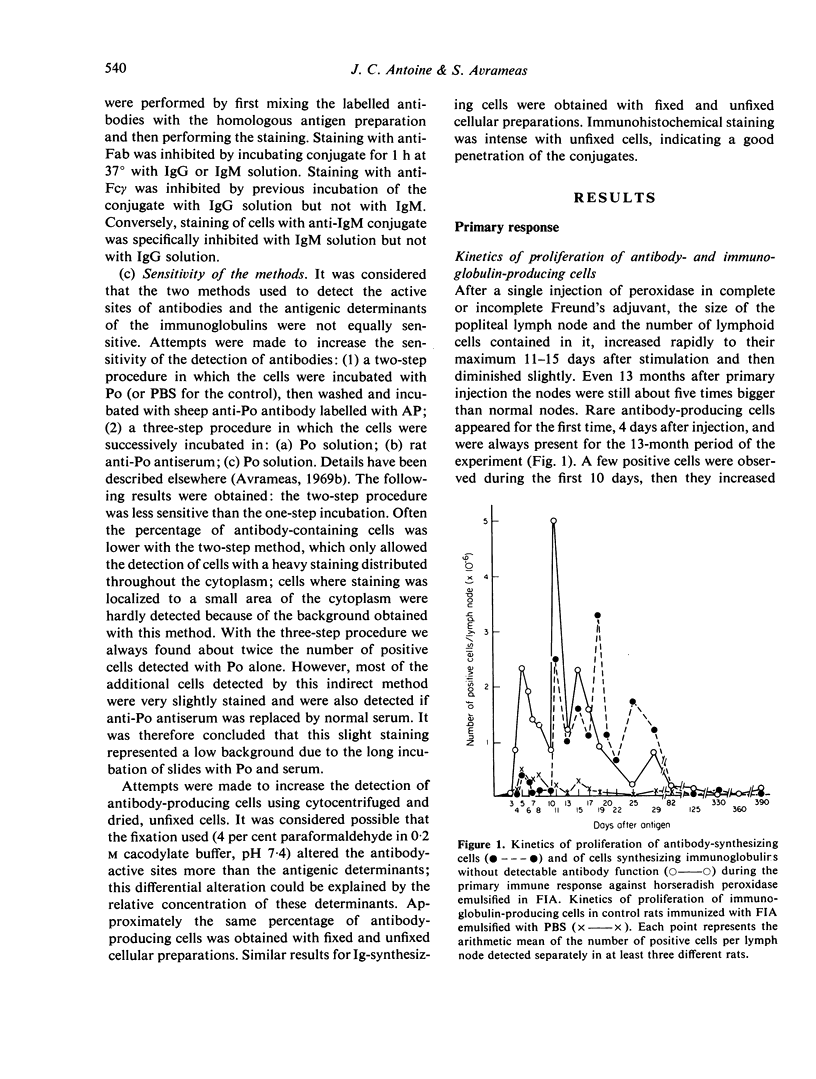
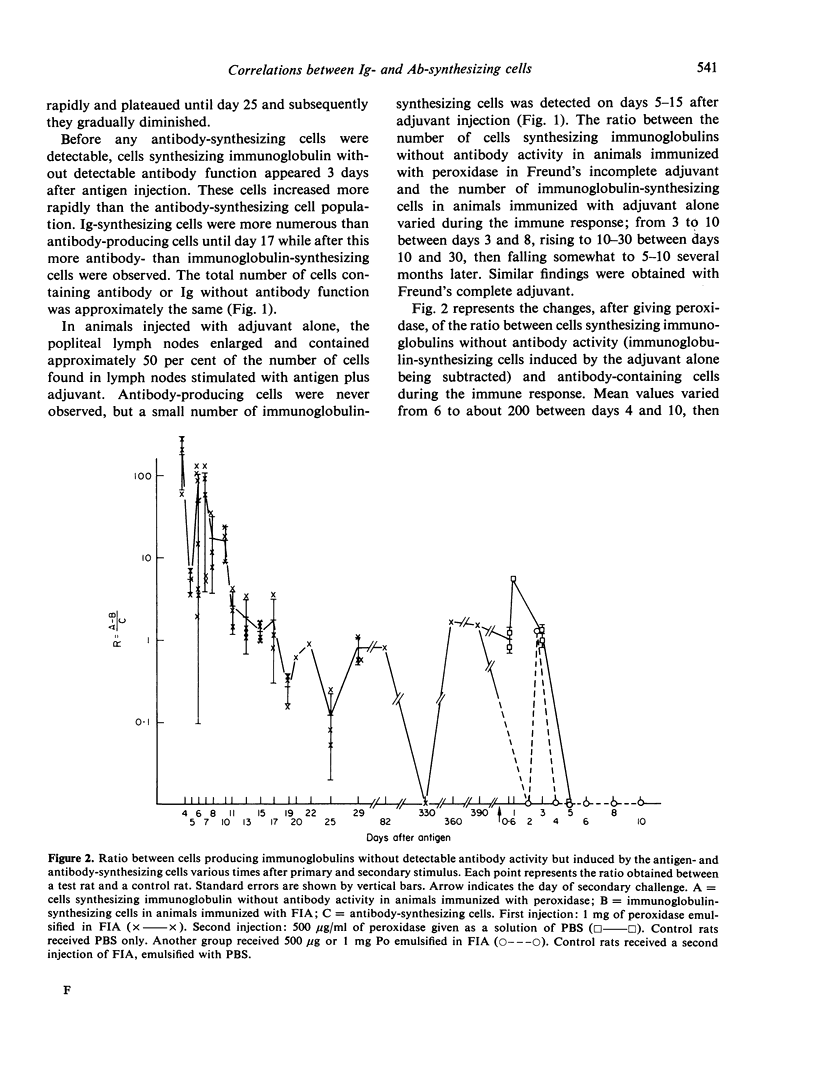
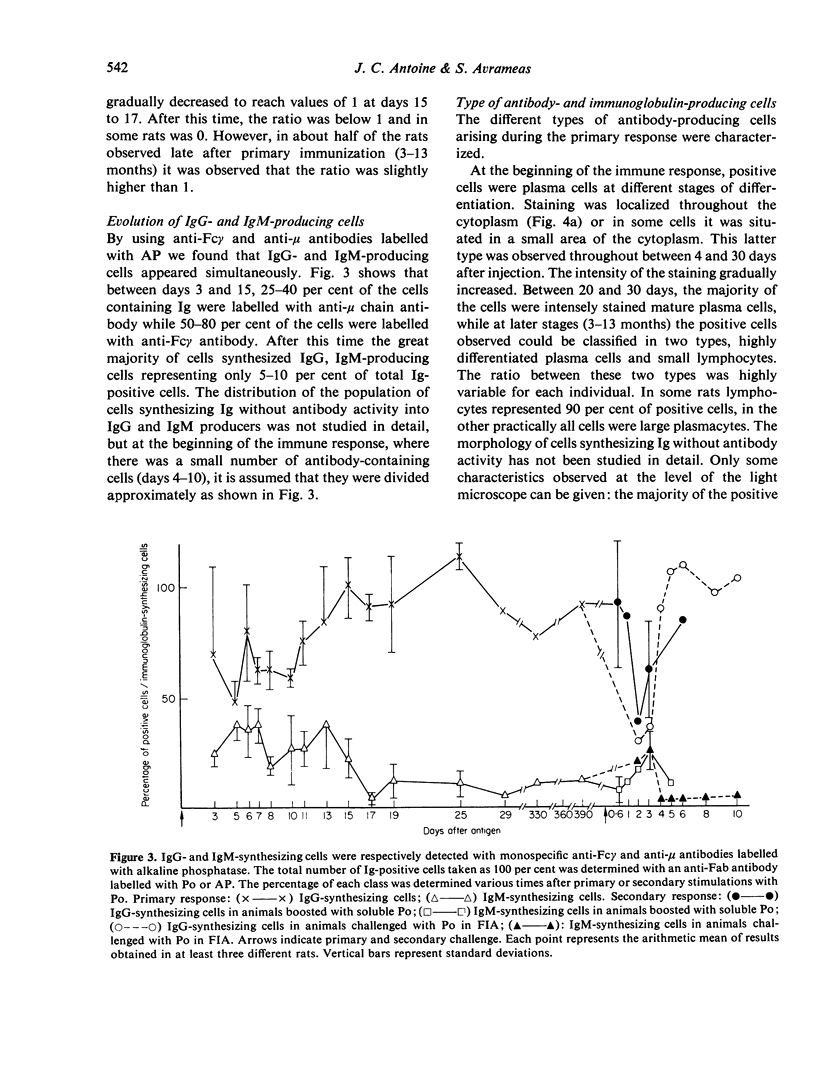
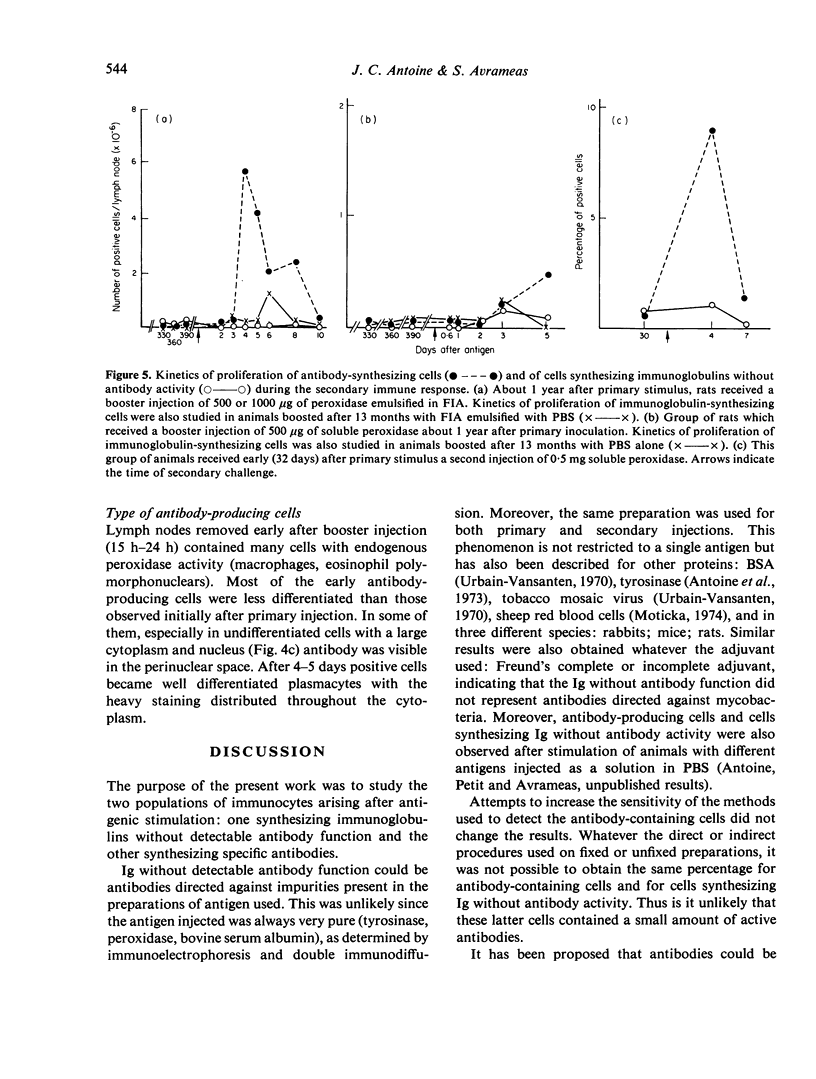
The development of cells synthesizing immunoglobulins without detectable antibody activity and of antibody-synthesizing cells was studied during primary and secondary immune responses of rats immunized with horseradish peroxidase. After primary immunization with peroxidase emulsified in Freund's complete or incomplete adjuvant, the first antibody-producing cells appeared 4 days after injection. They were preceded by cells synthesizing IgG and IgM without antibody function, appearing 3 days after giving antigen. The ratio between the latter and the former population of cells regularly decreased during the primary response. Seventy to 100 per cent of cells synthesizing immunoglobulins without antibody activity were induced by the antigen, the remainder being induced by the adjuvant. In both populations, the positive cells were always immature or mature plasmocytes. At various times after primary injection, animals received a booster inoculation of soluble peroxidase or of peroxidase emulsified in Freund's adjuvant. Antibody-producing cells, in early stages of differentiation, appeared between 2 and 3 days after challenge and were not preceded by cells synthesizing immunoglobulins without antibody function. These latter cells were reduced or absent after secondary challenge. Increasing the sensitivity of detection of active sites of antibodies, by using direct methods of staining with fixed or unfixed cells gave no increase of antibody-producing cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., HUMPHREY J. H. Formation of specific antibodies and gamma-globulin in vitro; a study of the synthetic ability of various tissues from rabbits immunized by different methods. Biochem J. 1958 Feb;68(2):252–261. doi: 10.1042/bj0680252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S. Etude de la formation des anticorps anti-tyrosinase au cours de la réponse immunitaire chez le lapin. I. Etude cellulaire. Ann Immunol (Paris) 1973 Feb;124(1):121–131. [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S. Surface immunoglobulins of rat immunocytes: quantitation and fate of cell-bound peroxidase-labeled antibody and Fab fragment. Eur J Immunol. 1974 Jul;4(7):468–474. doi: 10.1002/eji.1830040705. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Indirect immunoenzyme techniques for the intracellular detection of antigens. Immunochemistry. 1969 Nov;6(6):825–831. doi: 10.1016/0019-2791(69)90288-2. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Taudou B., Ternynck T. Specificity of antibodies synthetized by immunocytes as detected by immunoenzyme techniques. Int Arch Allergy Appl Immunol. 1971;40(2):161–170. doi: 10.1159/000230403. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bazin H., Beckers A., Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974 Jan;4(1):44–48. doi: 10.1002/eji.1830040112. [DOI] [PubMed] [Google Scholar]
- Cazenave P. A., Ternynck T., Avrameas S. Similar idiotypes in antibody-forming cells and in cells synthesizing immunoglobulins without detectable antibody function. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4500–4502. doi: 10.1073/pnas.71.11.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Anderson J., Bullock W. W. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur J Immunol. 1973 May;3(5):299–306. doi: 10.1002/eji.1830030509. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. In vitro induction of specific immune responses in the absence of serum: requirement for nonspecific T or B cell mitogens. Eur J Immunol. 1973 Sep;3(9):531–537. doi: 10.1002/eji.1830030902. [DOI] [PubMed] [Google Scholar]
- De Vos-Cloetens C., Minsart-Baleriaux V., Urbain-Vansanten G. Possible relationships between antibodies and non-specific immunoglobulins simultaneously induced after antigenic stimulation. Immunology. 1971 Jun;20(6):955–962. [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Antoine J. C., Stieber A., Avrameas S. Surface immunoglobulins of thymus and lymph node cells demonstrated by the peroxidase coupling technique. Lab Invest. 1972 Mar;26(3):253–261. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gurvitch A. E., Nikolaeva A. Changes in the synthesis of antibiodies and non-specific immunoglobulins in the spleen perfused in vitro. Immunology. 1971 Dec;21(6):915–924. [PMC free article] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- Kim Y. T., Siskind G. W. Studies on the control of antibody synthesis. VI. Effect of antigen dose and time after immunization on antibody affinity and heterogeneity in the mouse. Clin Exp Immunol. 1974 Jun;17(2):329–338. [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann W. D., Avrameas S. Cellular differentiation and antibody localization during the primary immune response in peroxidase stimulated lymph nodes of rat. Cell Immunol. 1972 Aug;4(4):425–441. doi: 10.1016/0008-8749(72)90044-5. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W. D., Avrameas S. Lymphocyte differentiation and antibody synthesis in the secondary immune response of peroxidase stimulated lymph nodes of rat. Cell Tissue Res. 1975;156(3):391–402. doi: 10.1007/BF00225367. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Avrameas S., Ternynck T. Intracellular distribution of antibody in immunocytes responding to primary challenge with horseradish peroxidase. Am J Pathol. 1973 May;71(2):239–260. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Avrameas S., Ternynck T. Intracellular distribution of antibody in immunocytes responding to secondary challenge with horseradish peroxidase. Am J Pathol. 1973 May;71(2):261–278. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Ternynck T., Avrameas S. A comparison of specific antibody and immunoglobulin synthesising immunocytes in peroxidase stimulated lymph nodes. Ann Immunol (Paris) 1974 Jan;125C(1-2):231–238. [PubMed] [Google Scholar]
- Miller H. R., Ternynck T., Avrameas S. Synthesis of antibody and immunoglobulins without detectable antibody function in cells responding to horseradish peroxidase. J Immunol. 1975 Feb;114(2 Pt 1):626–629. [PubMed] [Google Scholar]
- Mond J., Kim Y. T., Siskind G. W. Studies on the control of antibody synthesis. V. Effect of nonspecific modification of the magnitude of the immune response on the affinity of the antibody synthesized. J Immunol. 1974 Mar;112(3):1255–1263. [PubMed] [Google Scholar]
- Moticka E. J. The non-specific stimulation of immunoglobulin secretion following specific stimulation of the immune system. Immunology. 1974 Sep;27(3):401–412. [PMC free article] [PubMed] [Google Scholar]
- Oudin J., Cazenave P. A. Similar idiotypic specificities in immunoglobulin fractions with different antibody functions or even without detectable antibody function. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2616–2620. doi: 10.1073/pnas.68.10.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachmann K., Killander D., Wigzell H. Increase in intracellular immunoglobulins in the majority of splenic lymphoid cells after primary immunization. Eur J Immunol. 1974 Feb;4(2):138–145. doi: 10.1002/eji.1830040214. [DOI] [PubMed] [Google Scholar]
- Rubin A. S., Coons A. H. Specific heterologous enhancement of immune responses. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1665–1669. doi: 10.1073/pnas.68.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibienski R. J. Denaturation of lysozyme by Freund's complete adjuvant. J Immunol. 1973 Jul;111(1):114–120. [PubMed] [Google Scholar]
- Sidorova E. V., Chernomordik L. V., Gurvich A. E. A study of the nature of non-specific immunoglobulins by immunoexchange chromatography. Immunochemistry. 1975 May;12(5):397–401. doi: 10.1016/0019-2791(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Urbain-Vansanten G. Concomitant synthesis, in separate cells, of non-reactive immunoglobulins and specific antibodies after immunization with tobacco mosaic virus. Immunology. 1970 Nov;19(5):783–797. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]



