Abstract
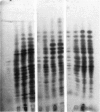
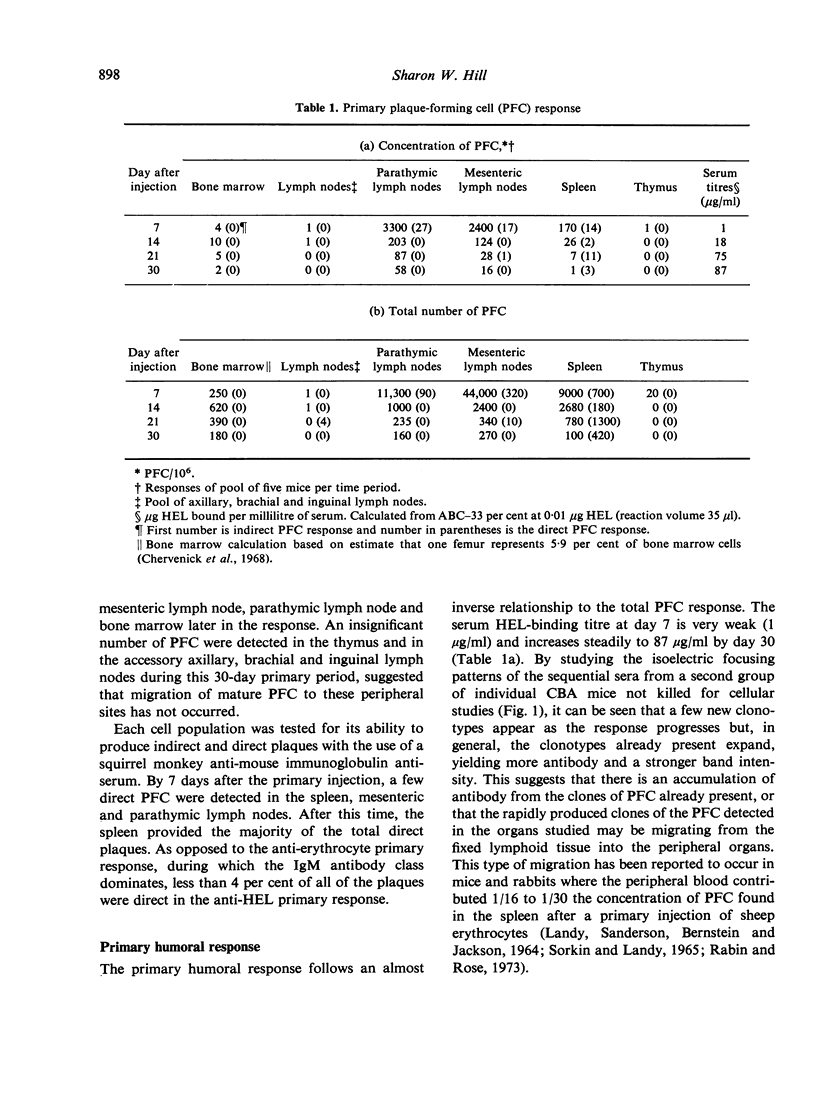
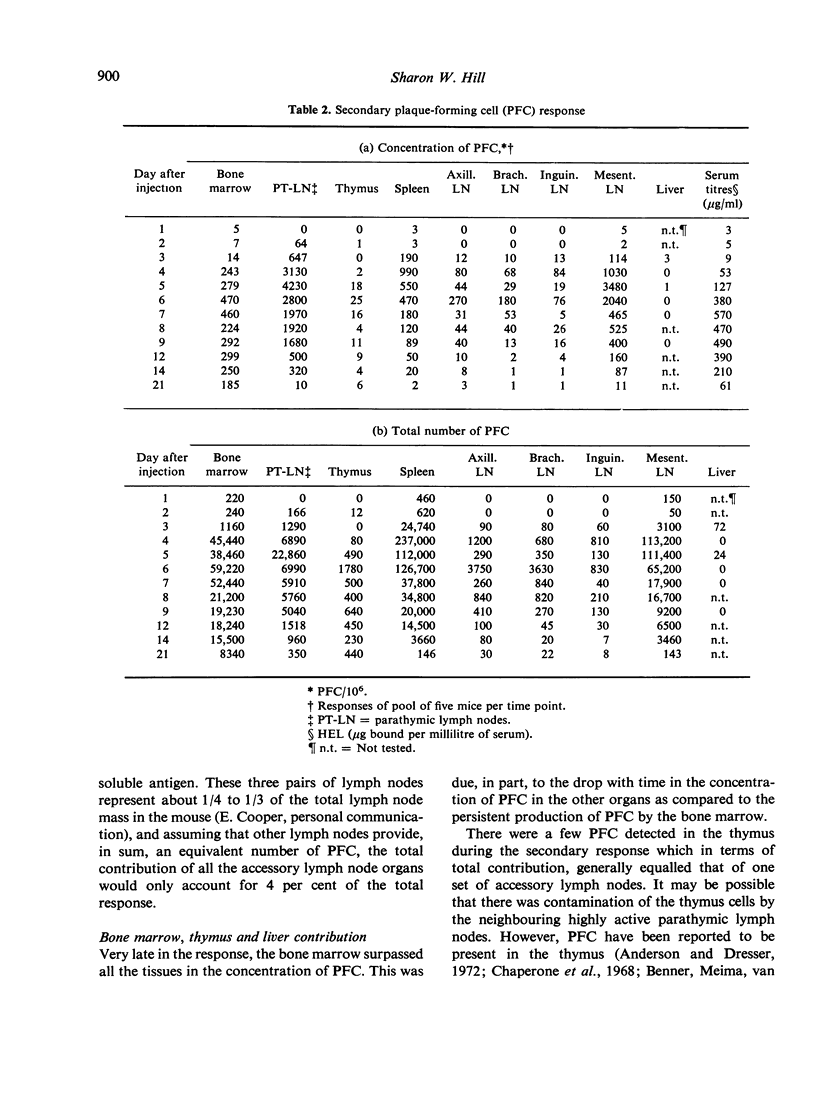
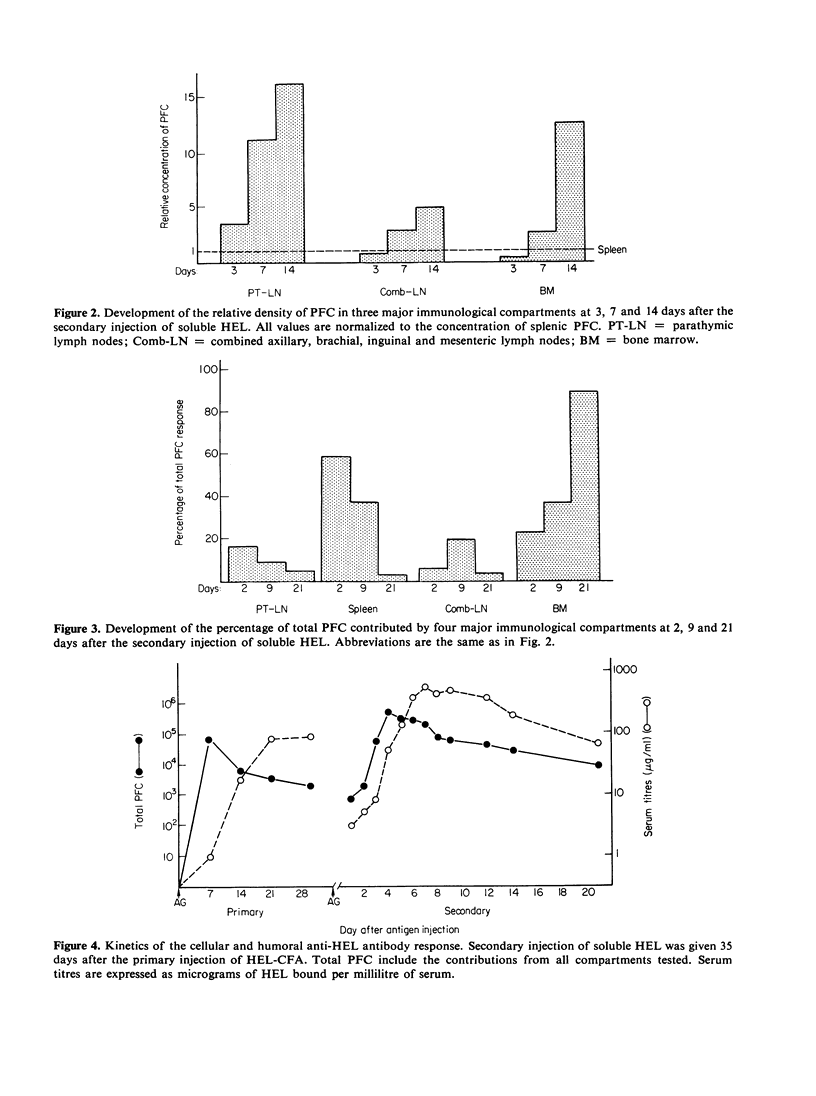
The distribution of plaque-forming cells (PFC) throughout the lymphoid system of CBA mice was followed with time after a primary intraperitoneal injection of hen egg white lysozyme emulsified in Freund's complete adjuvant (HEL-CFA) and after a secondary soluble injection. Throughout the primary response (predominantly IgG) and during the first week of the secondary response (exclusively IgG), the highest density of PFC was found in the draining parathymic lymph nodes, followed by the local spleen and mesenteric lymph nodes. The antibody-forming activity of the bone marrow increased as the immune response progressed, so that by the 3rd week of the secondary response this compartment provided the majority of the PFC. PFC first appeared in the accessory axillary, brachial or inguinal lymph nodes and in the thymus a few days after the secondary injection but accounted for only 1-5% of the total activity during the entire course of the secondary response. The specificity of the antibody produced in the spleen, parathymic and mesenteric lymph nodes was identical as judged by plaque inhibition by seven chemically related lysozymes which implies that these PFC were well mixed. It is postulated, therefore, that the change in distribution of PFC from an early local response to a general systemic response, and finally to a predominantly bone marrow response, was due to the migration of memory cells from the draining parathymic lymph nodes and spleen throughout the lymphoid system with an ultimate settling of the cells in the bone marrow.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., HUMPHREY J. H. Formation of specific antibodies and gamma-globulin in vitro; a study of the synthetic ability of various tissues from rabbits immunized by different methods. Biochem J. 1958 Feb;68(2):252–261. doi: 10.1042/bj0680252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKONAS B. A., WHITE R. G. Sites of antibody production in the guinea-pig; the relation between in vitro synthesis of anti-ovalbumin and gamma-globulin and distribution of antibody-containing plasma cells. Br J Exp Pathol. 1956 Feb;37(1):61–74. [PMC free article] [PubMed] [Google Scholar]
- Anderson H. R., Dresser D. W. The long-term distribution of antibody-forming cells. Eur J Immunol. 1972 Oct;2(5):410–413. doi: 10.1002/eji.1830020505. [DOI] [PubMed] [Google Scholar]
- Benner R., Meima F., Van der Meulen G. M., van Ewijk W. Antibody formation in mouse bone marrow. III. Effects of route of priming and antigen dose. Immunology. 1974 Nov;27(5):747–760. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., van der Meulen G. M. Antibody formation in mouse bone marrow. II. Evidence for a memory-dependent phenomenon. Cell Immunol. 1974 Jul;13(1):95–106. doi: 10.1016/0008-8749(74)90230-5. [DOI] [PubMed] [Google Scholar]
- Benner R., Meima F., van der Meulen G. M., van Muiswinkel W. B. Antibody formation in mouse bone marrow. I. Evidence for the development of plaque-forming cells in situ. Immunology. 1974 Feb;26(2):247–255. [PMC free article] [PubMed] [Google Scholar]
- Chaperon E. A., Selner J. C., Claman H. N. Migration of antibody-forming cells and antigen-sensitive precursors between spleen, thymus and bone marrow. Immunology. 1968 Apr;14(4):553–561. [PMC free article] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Daniels J. C., Weigle W. O. Antibody-producing cells in rabbits injected with soluble BSA. II. Kinetica and dose response. J Immunol. 1968 Dec;101(6):1230–1235. [PubMed] [Google Scholar]
- Eidinger D., Pross H. F. The immune response to sheep erythrocytes in the mouse. I. A study of the immunological events utilizing the plaque technique. J Exp Med. 1967 Jul 1;126(1):15–33. doi: 10.1084/jem.126.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. A., Wilkinson P. C., White R. G. Sites of biosynthesis of immunoglobulins in guinea-pigs immunized with bacteriophage phi-X174. Immunology. 1967 Dec;13(6):613–622. [PMC free article] [PubMed] [Google Scholar]
- Garvey J. S., Linker-Israeli M. Anti-SRBC plaque-forming cells in liver, spleen and hepatocyte cell suspensions of rabbits and mice. Immunol Commun. 1972;1(5):507–521. doi: 10.3109/08820137209022960. [DOI] [PubMed] [Google Scholar]
- Green I. Distribution of antibody-forming cells of different specificities in the lymph nodes and spleens of guinea pigs. J Exp Med. 1968 Oct 1;128(4):729–751. doi: 10.1084/jem.128.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Morris B., Moreno G. D., Bessis M. C. The ultrastructure and function of the cells in lymph following antigenic stimulation. J Exp Med. 1967 Jan 1;125(1):91–110. doi: 10.1084/jem.125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M., SANDERSON R. P., BERNSTEIN M. T., JACKSON A. L. ANTIBODY PRODUCTION BY LEUCOCYTES IN PERIPHERAL BLOOD. Nature. 1964 Dec 26;204:1320–1321. doi: 10.1038/2041320a0. [DOI] [PubMed] [Google Scholar]
- Leckband E., Boyse E. A. Immunocompetent cells among mouse thymocytes: a minor population. Science. 1971 Jun 18;172(3989):1258–1260. doi: 10.1126/science.172.3989.1258. [DOI] [PubMed] [Google Scholar]
- Lewis J. The theory of clonal mixing during growth. J Theor Biol. 1973 Apr;39(1):47–54. doi: 10.1016/0022-5193(73)90204-x. [DOI] [PubMed] [Google Scholar]
- Mauss H., Schmitt-Slomska J., Debré R. Modifications des ganglions parathymiques de la Souris aprés injection intrapéritionéale de formes L du streptocoque du groupe A. C R Acad Sci Hebd Seances Acad Sci D. 1974 Sep;279(10):879–882. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mellbye O. J. Antibody-producing cells in bone marrow and other lymphoid tissues during the primary immune response in mice. Int Arch Allergy Appl Immunol. 1971;40(2):248–255. doi: 10.1159/000230409. [DOI] [PubMed] [Google Scholar]
- Nash D. R. Direct and indirect plaque forming cells in extrapulmonary lymphoid tissue following local vs systemic injection of soluble antigen. Cell Immunol. 1973 Nov;9(2):234–241. doi: 10.1016/0008-8749(73)90074-9. [DOI] [PubMed] [Google Scholar]
- North J. R., Askonas B. A. Analysis of affinity of monoclonal antibody responses by inhibition of plaque-forming cells. Eur J Immunol. 1974 May;4(5):361–366. doi: 10.1002/eji.1830040511. [DOI] [PubMed] [Google Scholar]
- Rabin B. S., Rose N. R. Selective release of antibody forming cells into the blood. Cell Immunol. 1973 Jun;7(3):389–395. doi: 10.1016/0008-8749(73)90203-7. [DOI] [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Migration of small lymphocytes in adult mice demonstrated by parabiosis. Cell Tissue Kinet. 1974 Mar;7(2):137–150. doi: 10.1111/j.1365-2184.1974.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Röpke C., Hougen H. P., Everett N. B. Long-lived T and B lymphocytes in the bone marrow and thoracic duct lymph of the mouse. Cell Immunol. 1975 Jan;15(1):82–93. doi: 10.1016/0008-8749(75)90166-5. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Byers V. S. The X-Y-Z scheme of immunocyte maturation. 3. Early IgM memory and the nature of the memory cell. J Immunol. 1967 Apr;98(4):836–843. [PubMed] [Google Scholar]
- Sorkin E., Landy M. Antibody production by blood leucocytes. Experientia. 1965 Nov 15;21(11):677–680. doi: 10.1007/BF02144077. [DOI] [PubMed] [Google Scholar]
- Strober S., Dilley J. Maturation of B lymphocytes in the rat. I. Migration pattern, tissue distribution, and turnover rate of unprimed and primed B lymphocytes involved in the adoptive antidinitrophenyl response. J Exp Med. 1973 Dec 1;138(6):1331–1344. doi: 10.1084/jem.138.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER H. N., Jr, RAZZAK M. A., GAERTNER R. A., CAINE W. P., Jr, FEAGIN O. T. Removal of erythrocytes from the circulation. Arch Intern Med. 1962 Jul;110:90–97. doi: 10.1001/archinte.1962.03620190092014. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., Peacock M., Eltringham J. R. Regional lymph node irradiation: effect on local and distant generation of antibody forming cells. J Immunol. 1973 May;110(5):1300–1306. [PubMed] [Google Scholar]
- Werdelin O., McCluskey R. T., Witebsky E. In situ labeling of popliteal lymph node cells with tritiated thymidine in rats with allergic adrenalitis. Lab Invest. 1970 Aug;23(2):144–149. [PubMed] [Google Scholar]