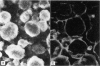
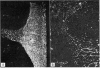
Abstract
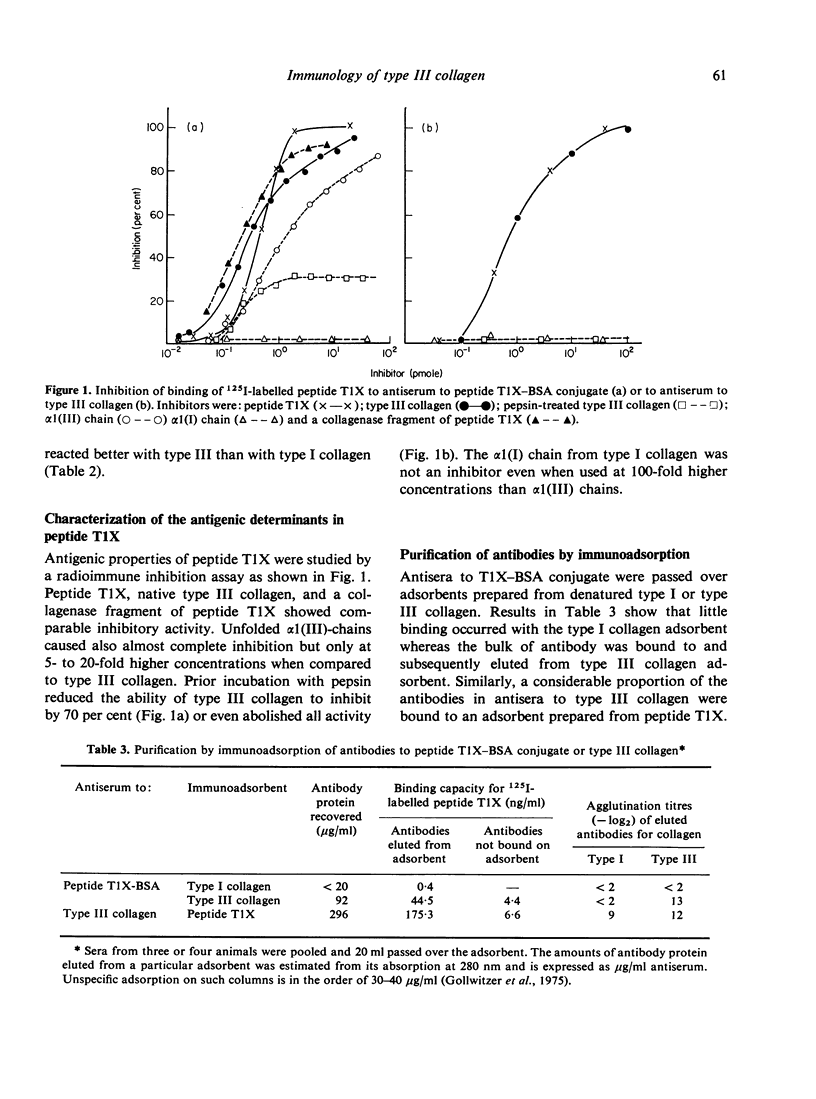
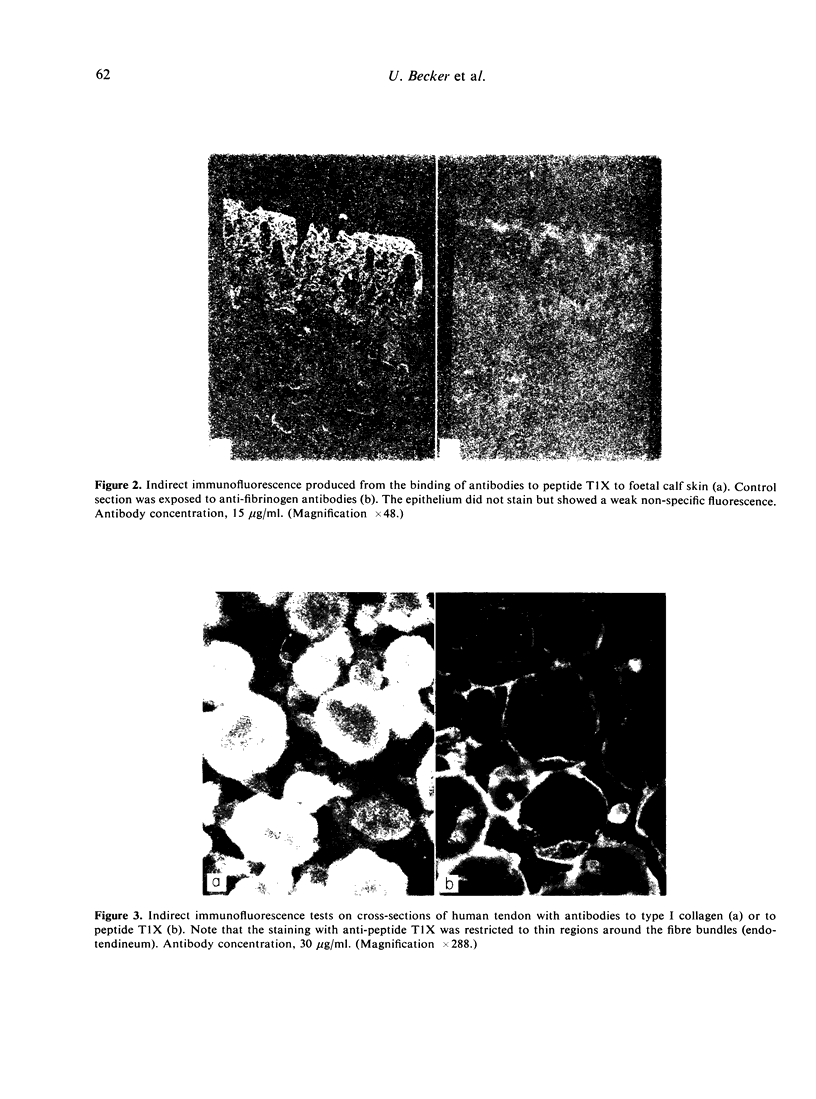
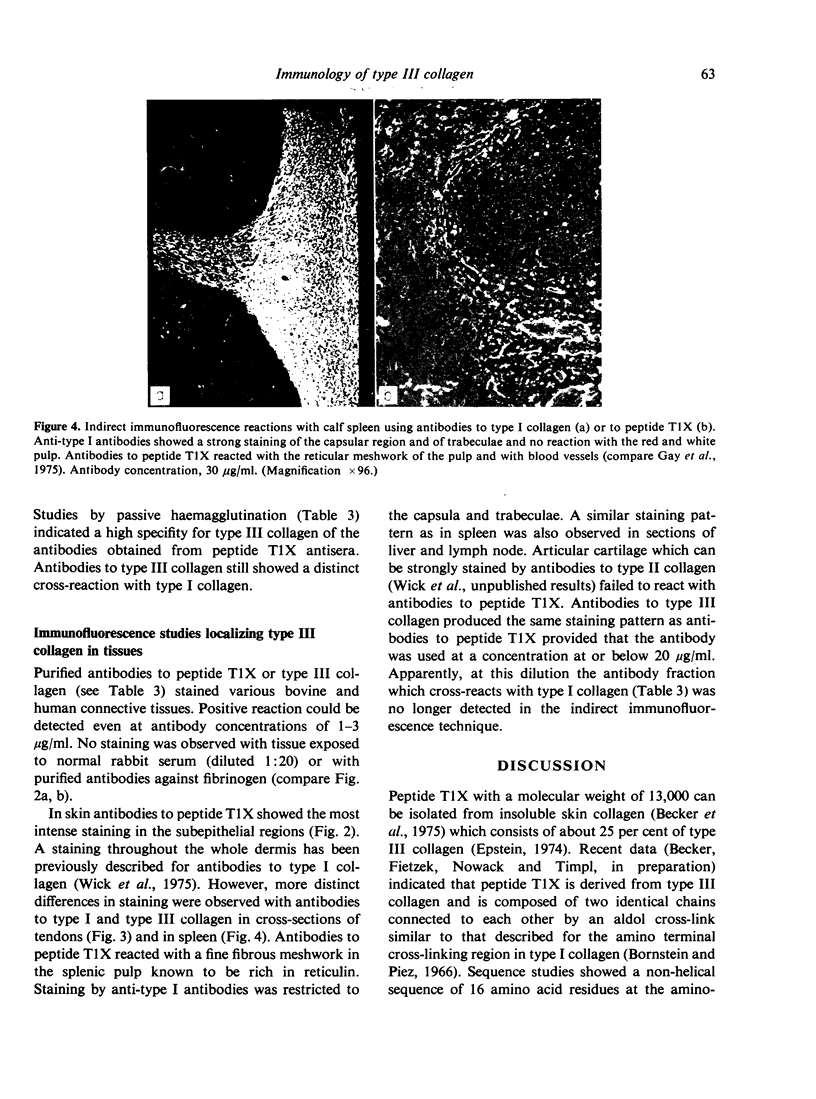
A cross-linked fragment (peptide T1X) with a molecular weight of 13,000 could be isolated from a tryptic digest of insoluble type III collagen of calf skin. Peptide T1X was conjugated on to bovine serum albumin by glutaraldehyde and used for immunization of rabbits. The antisera reacted in passive haemagglutination and radioimmune assay with peptide T1X, type III collagen and its constituent alpha1(III) chain. Little or no reaction was observed with type I collagen and alpha1(I) chain. While rabbit antisera to neutral salt-soluble type III Collagen also showed a strong binding for 125I-labelled peptide T1X much less reaction was observed with antisera to type I collagen. The antigenicity of type III collagen was largely destroyed by pepsin treatment suggesting that it resided in non-helical segments. A fragment of peptide T1X produced by digestion with collagenase retained antigenic activity. The data indicated that the aminoterminal region of type III collagen contains strong antigenic determinants located in a non-helical sequence of about sixteen amino acids. Antibodies to these antigenic determinants were purified and rendered specific for type III collagen by immunoadsorption. The antibodies stained in indirect immunofluorescence tests particularly those regions in various connective tissues which are rich in reticulin fibres. Different staining patterns were observed with antibodies to type I collagen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelmann B. C., Gentner G. J., Hopper K. A sensitive radioimmunoassay for collagen. J Immunol Methods. 1973 Dec;3(4):319–336. doi: 10.1016/0022-1759(73)90034-3. [DOI] [PubMed] [Google Scholar]
- Beil W., Timpl R., Furthmayr H. Conformation dependence of antigenic determinants on the collagen molecule. Immunology. 1973 Jan;24(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Immunochemistry of collagens and procollagens. Int Rev Connect Tissue Res. 1976;7:61–99. doi: 10.1016/b978-0-12-363707-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Gollwitzer R., Hahn E., Nowack H., Timpl R. Immunochemistry of bovine fibrinogen--I. immunogenic activity and diversity of antigenic determinants of reduced and carboxymethylated alpha, beta and gamma chains. Immunochemistry. 1975 Nov;12(11):893–897. doi: 10.1016/0019-2791(75)90247-5. [DOI] [PubMed] [Google Scholar]
- Hahn E., Timpl R., Miller E. J. Demonstration of a unique antigenic specificity for the collagen alpha1 (II) chain from cartilaginous tissue. Immunology. 1975 Mar;28(3):561–568. [PMC free article] [PubMed] [Google Scholar]
- Hahn E., Timpl R., Miller E. J. The production of specific antibodies to native collagens with the chain compositions, (alpha1(I))3, (alpha1(II))3, and (alpha1(I))2alpha 2. J Immunol. 1974 Jul;113(1):421–423. [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- King T. P., Spencer M. Structural studies and organic ligand-binding properties of bovine plasma albumin. J Biol Chem. 1970 Nov 25;245(22):6134–6148. [PubMed] [Google Scholar]
- Lotter H., Timpl R. Antigenic structure of the cyanogen bromide peptide F-CB3 from fibrinogen alpha-chain. Eur J Biochem. 1975 Dec 1;60(1):221–226. doi: 10.1111/j.1432-1033.1975.tb20994.x. [DOI] [PubMed] [Google Scholar]
- Maoz A., Fuchs S., Sela M. Immune response to the collagen-like synthetic ordered polypeptide (L-Pro-Gly-L-Pro)n. Biochemistry. 1973 Oct 9;12(21):4238–4245. doi: 10.1021/bi00745a031. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Miller E. J. A review of biochemical studies on the genetically distinct collagens of the skeletal system. Clin Orthop Relat Res. 1973 May;(92):260–280. doi: 10.1097/00003086-197305000-00024. [DOI] [PubMed] [Google Scholar]
- Nowack H., Hahn E., Timpl R. Specificity of the antibody response in inbred mice to bovine type I and type II collagen. Immunology. 1975 Oct;29(4):621–628. [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Pontz B., Meigel W., Rauterberg J., Kühn K. Localization of two species specific antigenic determinants on the peptide chains of calf skin collagen. Eur J Biochem. 1970 Sep;16(1):50–54. doi: 10.1111/j.1432-1033.1970.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Wick G., Furthmayr H., Timpl R. Purified antibodies to collagen: an immunofluorescence study of their reaction with tissue collagen. Int Arch Allergy Appl Immunol. 1975;48(5):664–679. doi: 10.1159/000231354. [DOI] [PubMed] [Google Scholar]
- Wright R., Alp M. H. Autoantibodies to reticulin in patients with various gastrointestinal diseases and their relationship to immunoglobulins and dietary antibodies. Gut. 1971 Oct;12(10):858–859. [PubMed] [Google Scholar]