Abstract
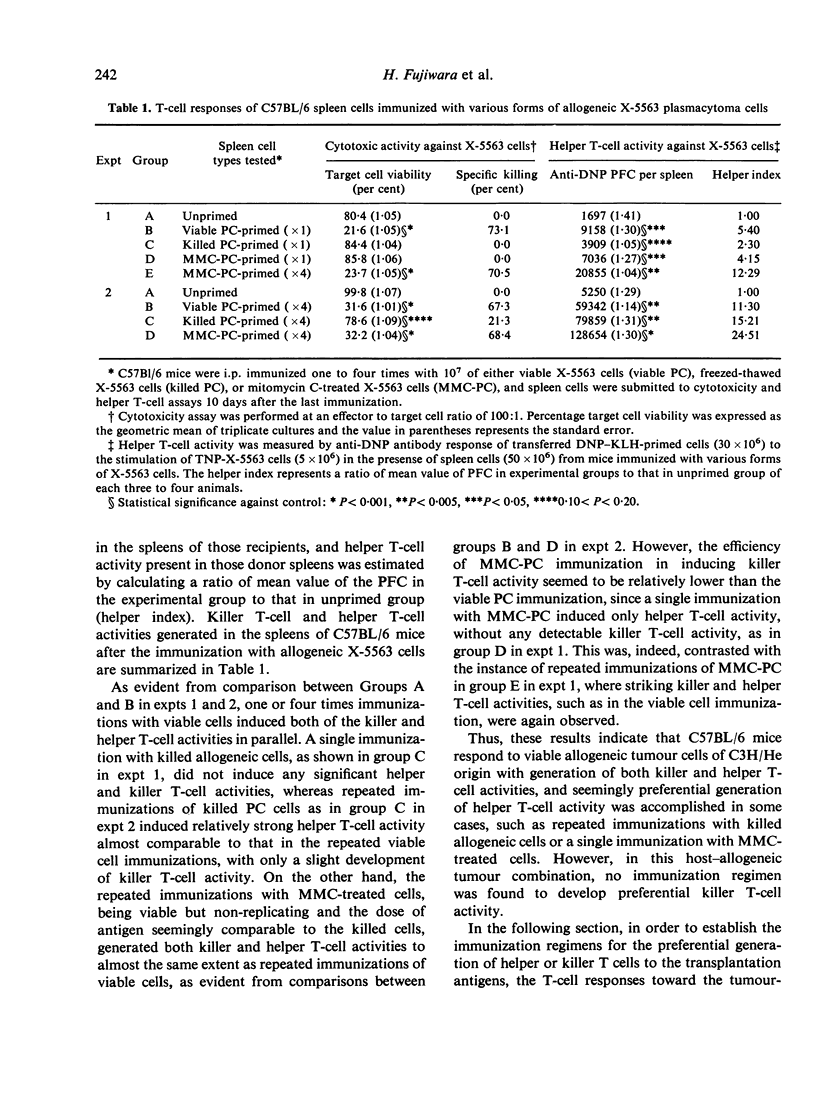
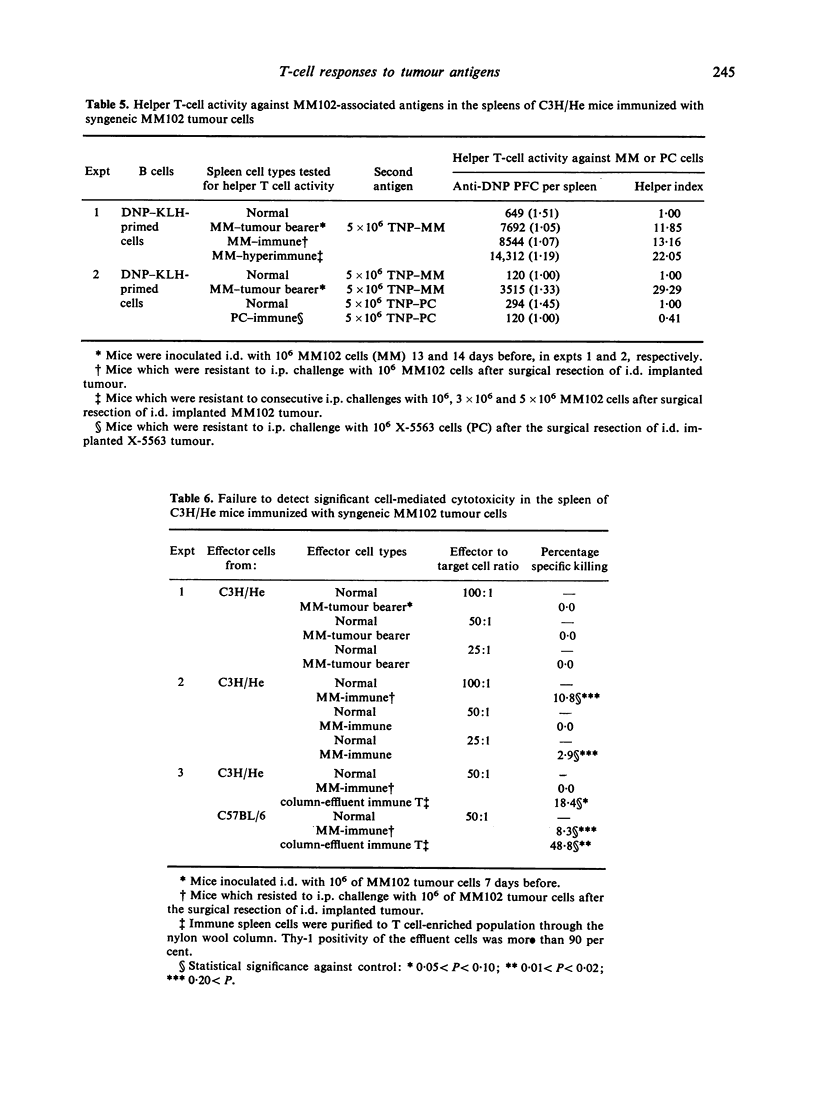
Induction of killer and helper T-cell activities towards transplantation antigens of two tumour cell lines was analysed in the allogeneic and syngeneic host combinations. The lymphoid cells from C57BL/6 mice immunized with allogeneic viable or mitomycin C-treated X-5563 plasmacytoma cells derived from C3H/He mice revealed both killer and helper T-cell activities against alloantigens, whereas cells from mice immunized with tumour cells killed by a freezing and thawing procedure revealed predominantly helper T-cell activity. On the other hand, when C3H/He mice were immunized with viable syngeneic X-5563 plasmacytoma or MM102 mammary tumour cells, the former generated preferentially killer T-cell activity, whereas the latter induced predominantly helper T-cell activity against tumour-associated transplantation antigens. Thus, immunization with transplantation antigen(s) does not always induce both helper and killer T-cell activities in parallel, but a certain antigenic system induces predominantly one type of T-cell response, thus indicating that two distinct subsets of helper and killer T cells against the transplantation antigen(s) can be raised independently without an absolute requirement of collaboration between these different T-cells subsets.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Lennox E. S. Cooperation and cell-mediated cytotoxicity as functions of two subsets of T cells. J Immunol. 1974 Nov;113(5):1553–1561. [PubMed] [Google Scholar]
- Hamaoka T., Yamaskita U., Takami T., Kitagawa M. The mechanism of tolerance induction in thymus-derived lymphocytes; I. intracellular inactivation of hapten-reactive helper T lymphocytes by hapten-nonimmunogenic copolymer of D-amino acids. J Exp Med. 1975 Jun 1;141(6):1308–1328. doi: 10.1084/jem.141.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Sudo H. Evaluation of cell damage in immune reactions by release of radioactivity from 3 H-uridine labeled cells. Gan. 1971 Apr;62(2):139–143. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klein J., Forman J., Hauptfeld V., Egorov I. K. Immunogenetic analysis of H-2 mutations. III. Genetic mapping and involvement in immune reactions of the H-2ka mutation. J Immunol. 1975 Sep;115(3):716–718. [PubMed] [Google Scholar]
- Klein J., Livnat S., Hauptfeld V., Jerábek L., Weissman I. Production of anti-H-2 antibodies in thymectomized mice. Eur J Immunol. 1974 Jan;4(1):41–44. doi: 10.1002/eji.1830040111. [DOI] [PubMed] [Google Scholar]
- Philpott G. W., Bower R. J., Parker C. W. Selective cytotoxicity in a hapten substituted cell culture model system. J Immunol. 1973 Sep;111(3):930–937. [PubMed] [Google Scholar]
- Takizawa K., Yamamoto T., Mitsui H. Transplantion resistance and potent antibody production induced in C3H/He mice against C3H/He mammary tumor by continuous infusion of solubilized antigen. Gan. 1974 Dec;65(6):541–544. [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M., Nossal G. J. T-cell-mediated immune responses induced in vitro: a probe for allograft and tumor immunity. Transplant Rev. 1973;17(0):3–36. doi: 10.1111/j.1600-065x.1973.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Wernet D., Lilly F. Genetic regulation of the antibody response to H-2Db alloantigens in mice. I. Differences in activation of helper T cells in C57BL/10 and BALB/c congenic strains. J Exp Med. 1975 Mar 1;141(3):573–583. doi: 10.1084/jem.141.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


