Abstract
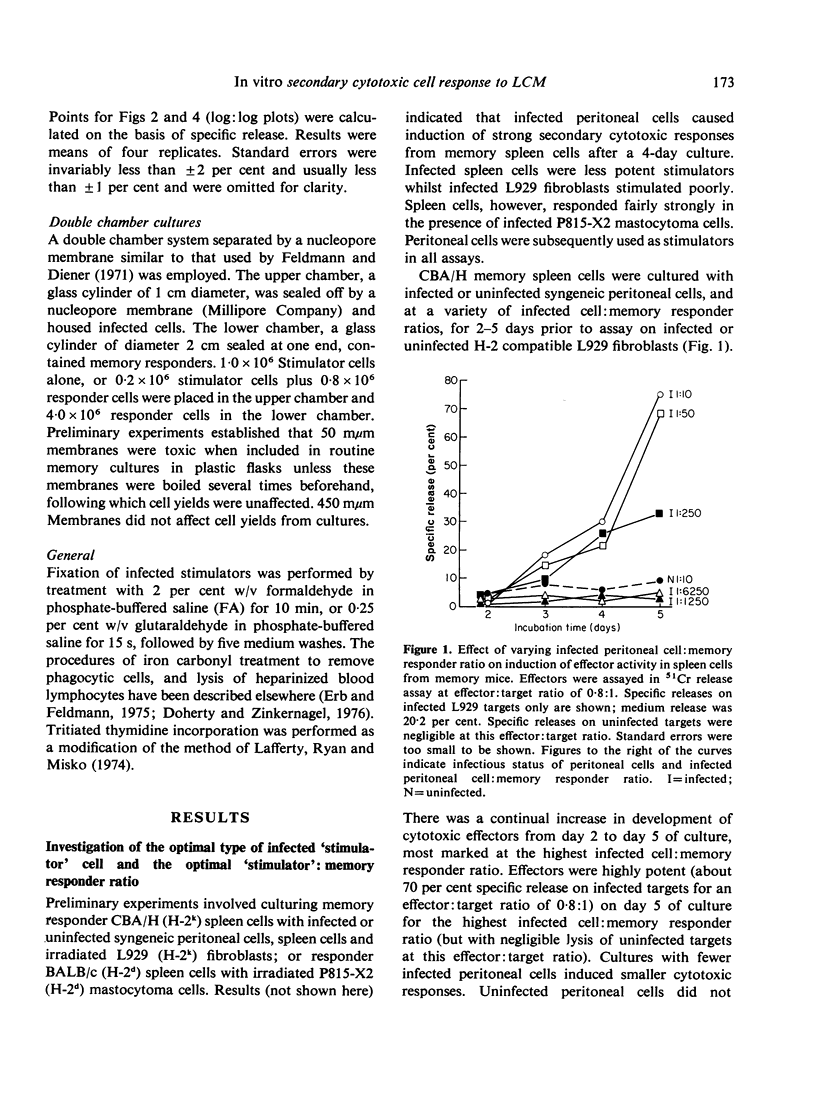
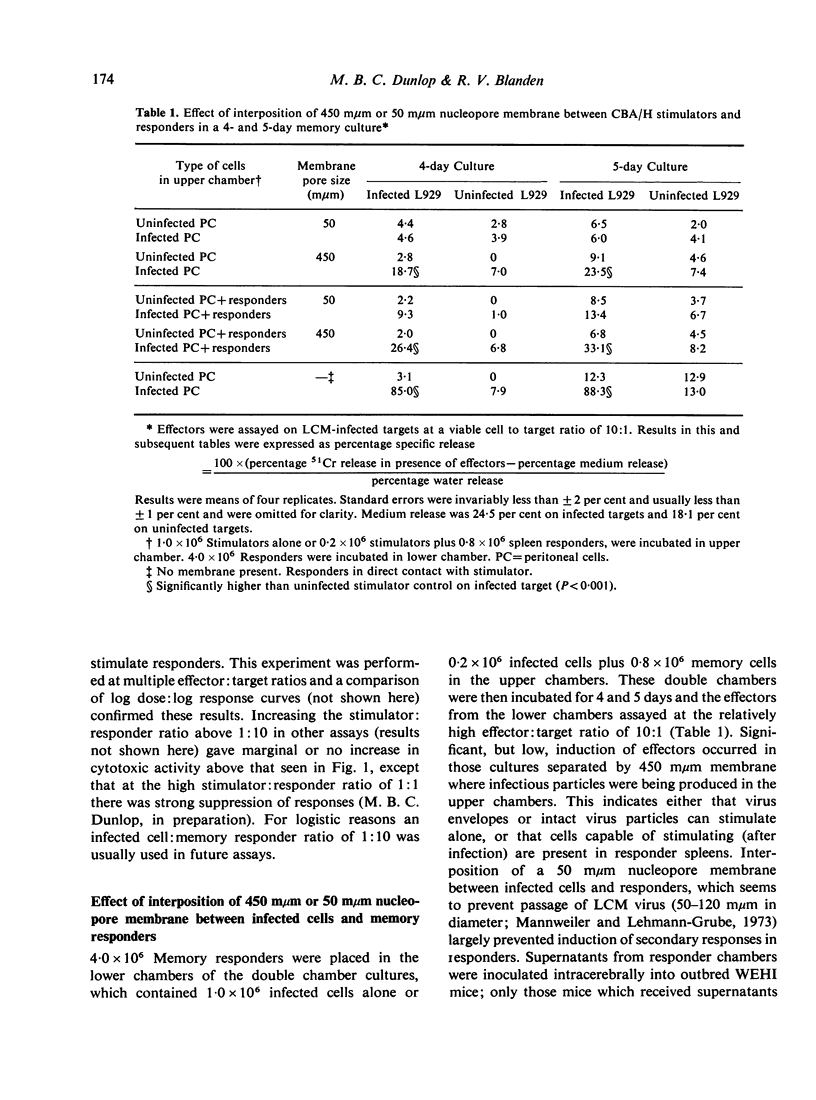
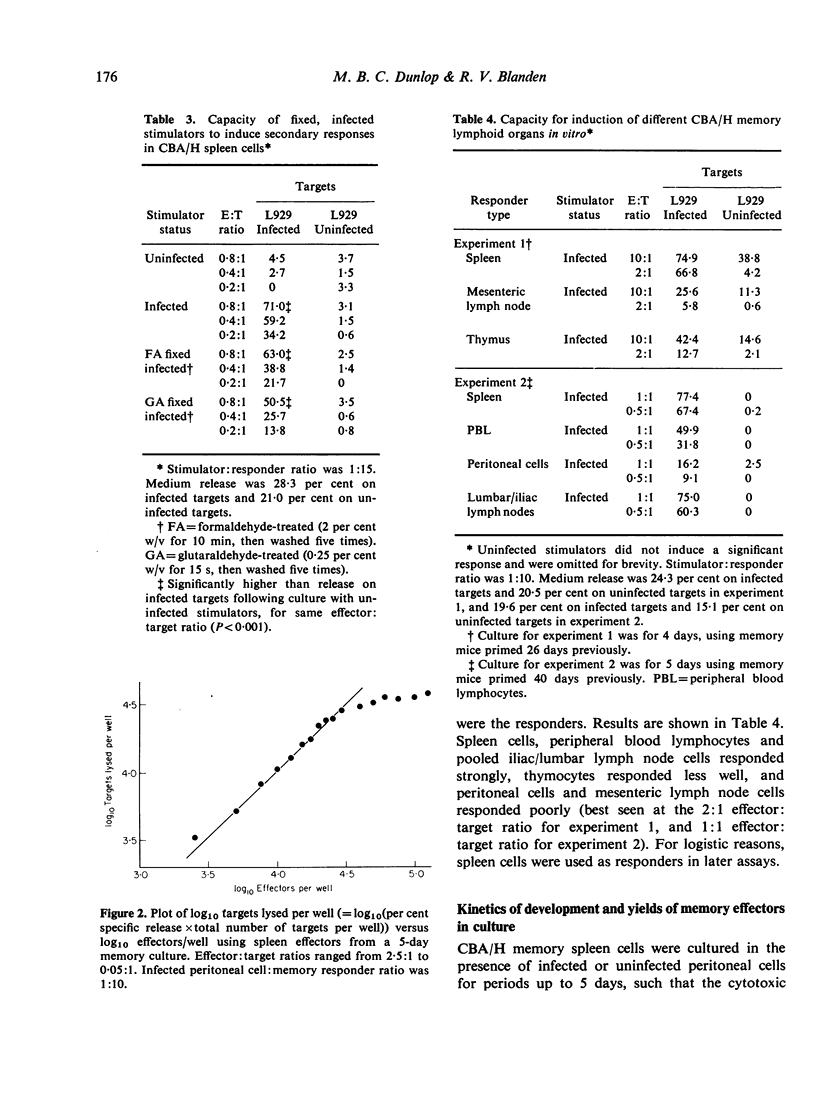
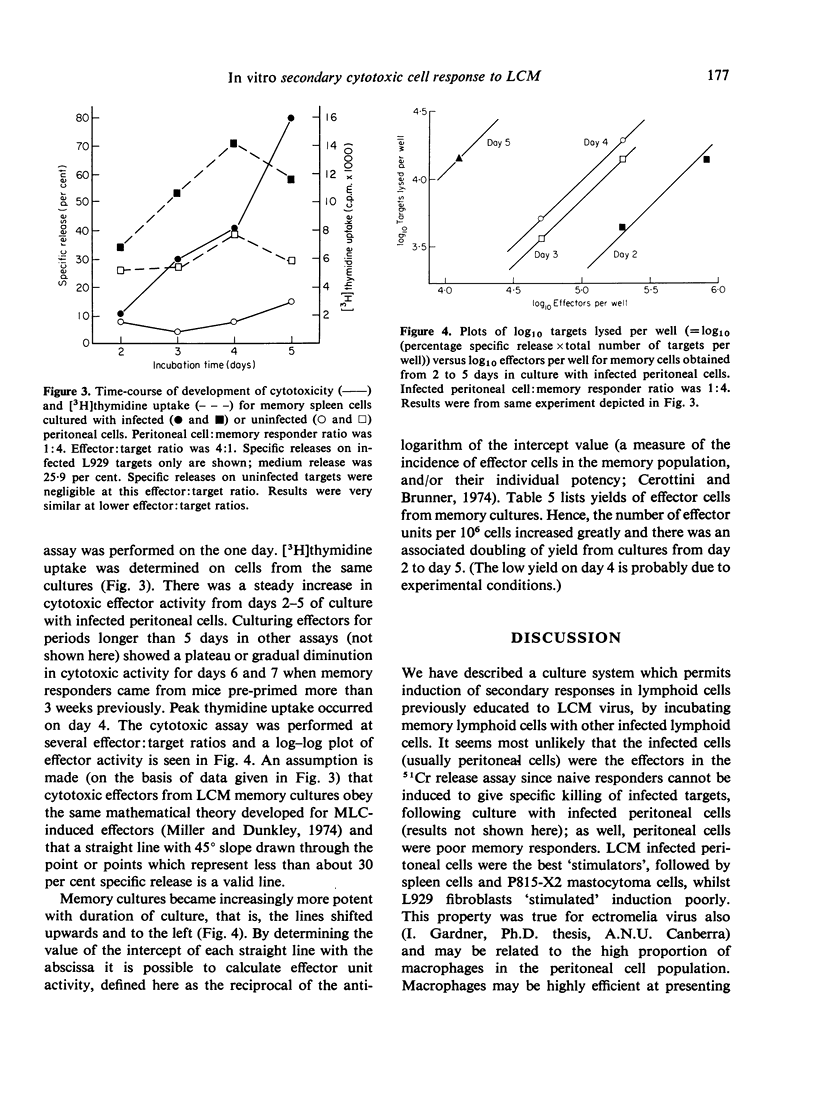
Secondary (memory) cell-mediated cytotoxic responses in lymphoid cells from CBA/H mice pre-primed with lymphocytic choriomeningitis virus (LCM) 5-7 weeks previously were induced by culturing these cells in vitro with syngeneic, infected peritoneal cells at 37 degrees for periods of up to 5 days. Cytotoxic effectors were assayed against LCM infected, H-2 compatible target cells in a 51Cr release assay. Response was greater with a higher ratio (1:10) of infected peritoneal cells:pre-primed cells than with lower ratios (e.g. 1:250). Separating responders from infected cells by a 450 mmum nucleopore membrane (coarse enough to allow passage of virus particles) still permitted induction of a secondary response whilst interposition of a 50 mmum nucleopore membrane (which apparently prevented transit of virus particles) virtually abolished the secondary response. Removal of phagocytic cells from responders prior to setting up memory cultures greatly reduced responders' capacity to be induced. Fixed, infected stimulators still induced strong secondary responses. Secondary response was maximal with spleen cells, peripheral blood lymphocytes, or pooled iliac and lumbar lymph node cells. Thymocytes responded less well, whilst mesenteric lymphoid cells and peritoneal cells gave minimal responses. Effector cells from memory cultures killed targets with single-hit kinetics and a rectilinear log effectors: log targets lysed relation held. Memory spleen cells developed increasing cytolytic activity from 2 to 5 days in culture. Memory-generated effectors were markedly potent by day 5, e.g. giving 70 per cent specific release at a killer:target ratio of 0-8:1. Peak DNA synthesis occurred on day 4. We conclude that memory effectors as a population differ in kinetics and potency from effectors obtained by primary viral challenge in the mouse.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennert G., Lennox E. S. Cooperation and cell-mediated cytotoxicity as functions of two subsets of T cells. J Immunol. 1974 Nov;113(5):1553–1561. [PubMed] [Google Scholar]
- Engers H. D., Thomas K., Cerottini J. C., Brunner K. T. Generation of cytotoxic T lymphocytes in vitro. V. Response of normal and immune spleen cells to subcellular alloantigens. J Immunol. 1975 Aug;115(2):356–360. [PubMed] [Google Scholar]
- Erb P., Feldmann M. Role of macrophages in in vitro induction of T-helper cells. Nature. 1975 Mar 27;254(5498):352–354. doi: 10.1038/254352a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Diener E. Antibody-mediated suppression of the immune response in vitro. 3. Low zone tolerance in vitro. Immunology. 1971 Sep;21(3):387–404. [PMC free article] [PubMed] [Google Scholar]
- Gardner I., Bowern N. A., Blanden R. V. Cell-mediated cytotoxicity against ectromelia virus-infected target cells. I. Specificity and kinetics. Eur J Immunol. 1974 Feb;4(2):63–67. doi: 10.1002/eji.1830040202. [DOI] [PubMed] [Google Scholar]
- Häyry P., Andersson L. C. Generation of T memory cells in one-way mixed lymphocyte culture. II. Anamnestic responses of "secondary" lymphocytes. Scand J Immunol. 1974;3(6):823–832. doi: 10.1111/j.1365-3083.1974.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Unanue E. R. Critical role of determinant presentation in the induction of specific responses in immunocompetent lymphocytes. J Exp Med. 1973 Apr 1;137(4):967–990. doi: 10.1084/jem.137.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Thomssen R. Target cell-dependent T cell-mediated lysis of vaccinia virus-infected cells. Eur J Immunol. 1975 Apr;5(4):245–251. doi: 10.1002/eji.1830050405. [DOI] [PubMed] [Google Scholar]
- Lafferty K., Ryan M., Misko I. An improved system for the assay of stimulation in mouse mixed leucocyte cultures. J Immunol Methods. 1974 Mar;4(2):263–273. doi: 10.1016/0022-1759(74)90069-6. [DOI] [PubMed] [Google Scholar]
- Leclerc J. C., Gomard E., Levy J. P. Cell-mediated reaction against tumors induced by oncornaviruses. I. Kinetics and specificity of the immune response in murine sarcoma virus (MSV)-induced tumors and transplanted lymphomas. Int J Cancer. 1972 Nov;10(3):589–601. doi: 10.1002/ijc.2910100318. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Dunkley M. Quantitative analysis of the 51Cr release cytotoxicity assay for cytotoxic lymphocytes. Cell Immunol. 1974 Nov;14(2):284–302. doi: 10.1016/0008-8749(74)90212-3. [DOI] [PubMed] [Google Scholar]
- Plata F., Cerottini J. C., Brunner K. T. Primary and secondary in vitro generation of cytolytic T lymphocytes in the murine sarcoma virus system. Eur J Immunol. 1975 Apr;5(4):227–233. doi: 10.1002/eji.1830050402. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M. Secondary cytotoxic tumor immune response induced in vitro. J Immunol. 1974 May;112(5):1718–1725. [PubMed] [Google Scholar]
- Wagner H., Wyss C. Cell-mediated immune responses in vitro. V. A comparative study of in vitro immunogenicity of splenic lymphocytes, neoplastic lymphoid cells and fibroblats. Eur J Immunol. 1973 Sep;3(9):549–555. doi: 10.1002/eji.1830030905. [DOI] [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: functional aspects of antigen handling by macrophages. J Immunol. 1974 Feb;112(2):746–755. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Characteristics of the interaction in vitro between cytotoxic thymus-derived lymphocytes and target monolayers infected with lymphocytic choriomeningitis virus. Scand J Immunol. 1974;3(3):287–294. doi: 10.1111/j.1365-3083.1974.tb01259.x. [DOI] [PubMed] [Google Scholar]


