Abstract
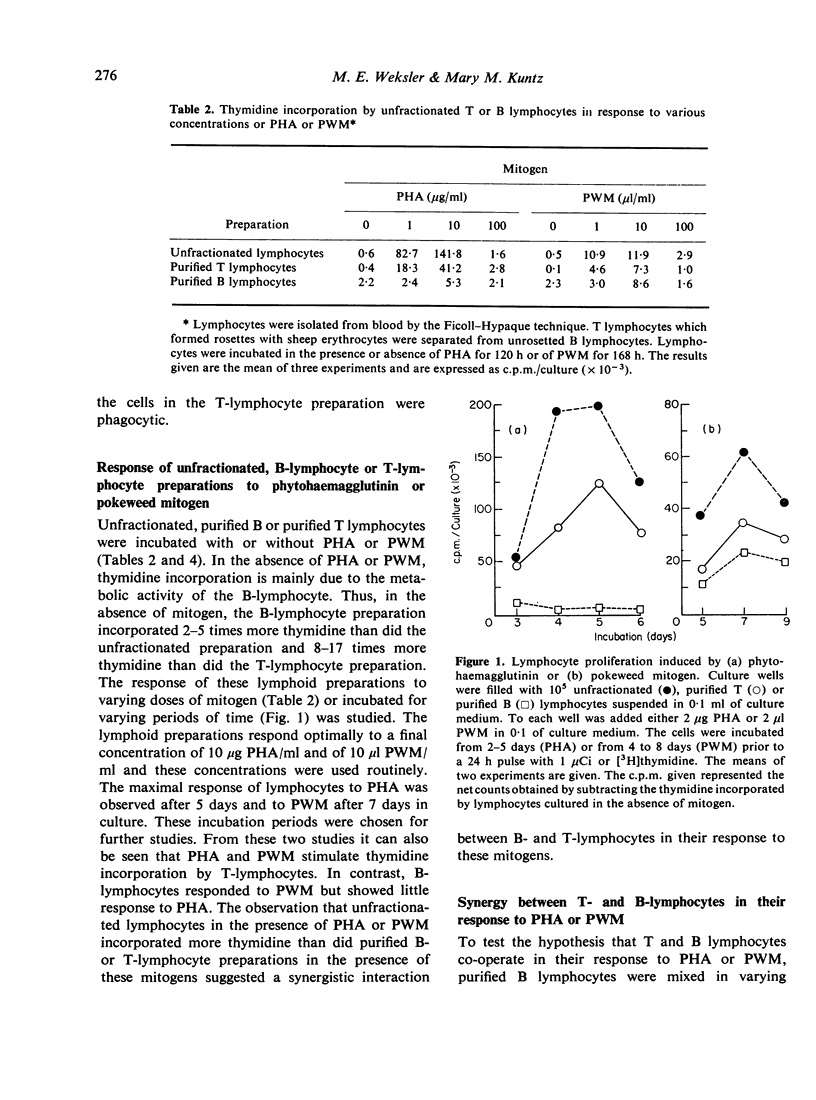
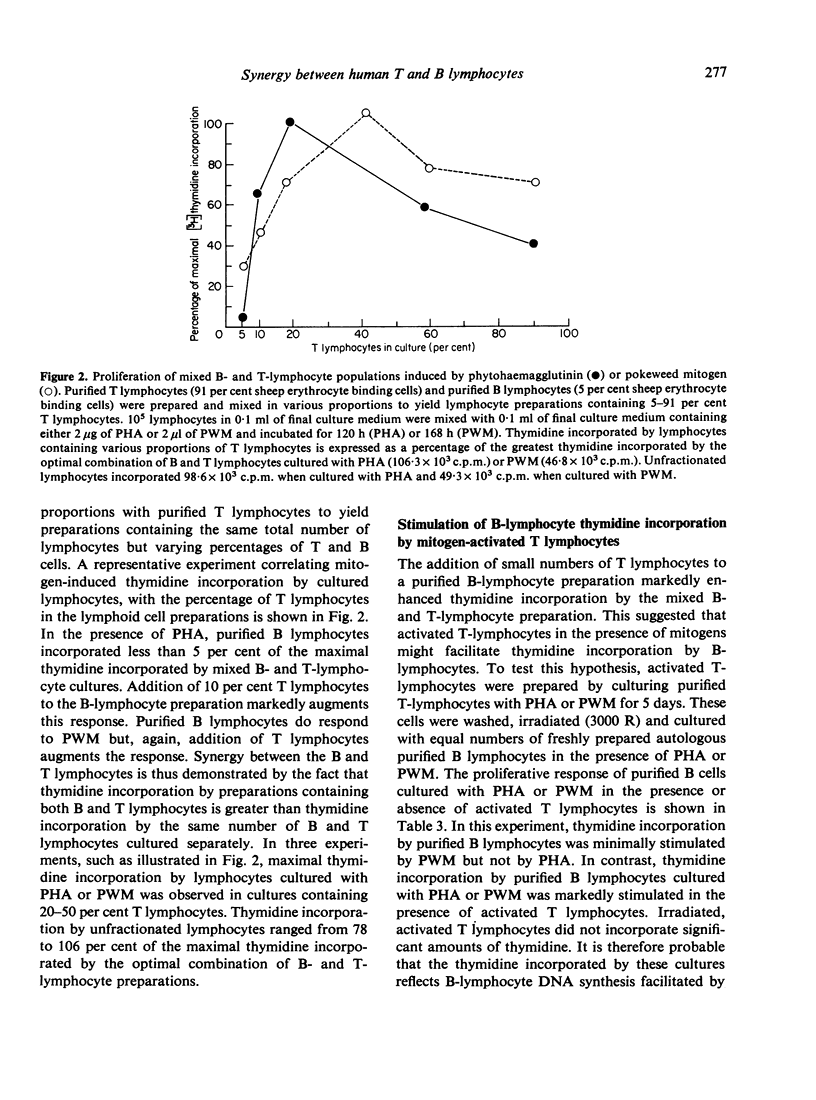
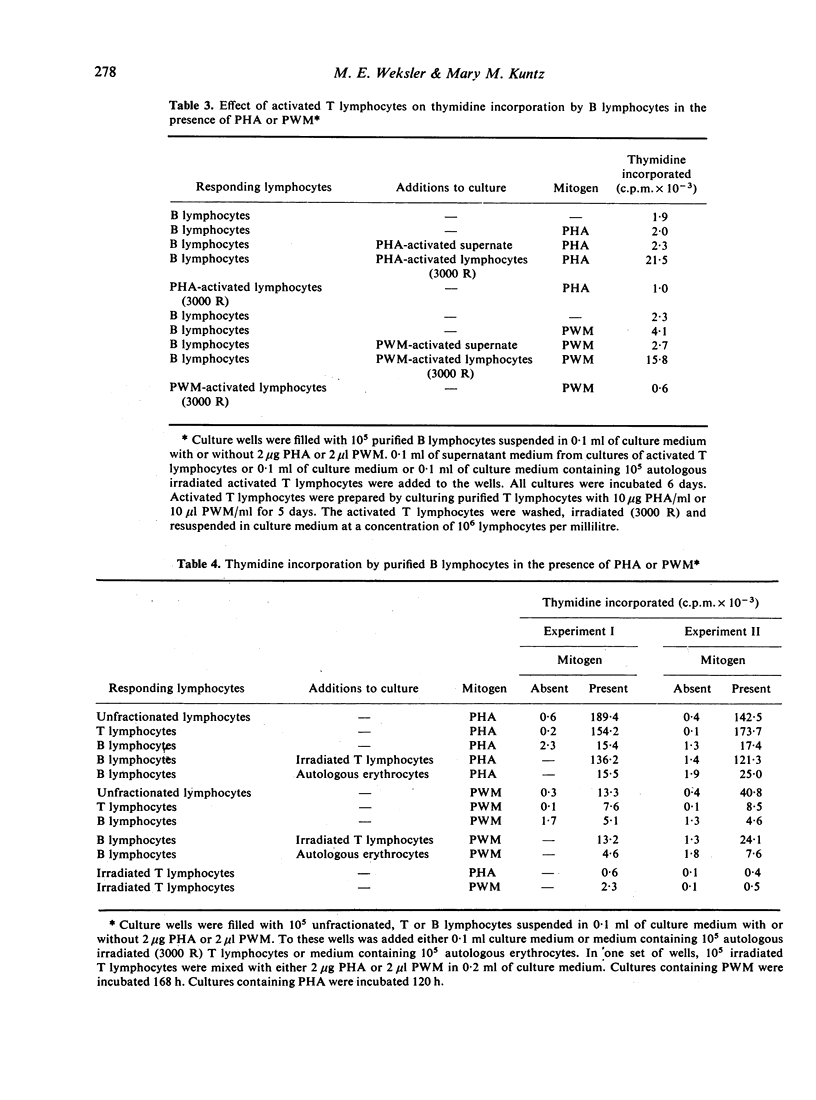
Human B- and T-lymphocyte preparations were isolated by separating T lymphocytes that formed rosettes with sheep erythrocytes from unrosetted B lymphocytes. Pokeweed mitogen stimulates the proliferation of both B- and T-lymphocyte preparations. In contrast, phytohaemagglutinin stimulates little or no proliferation of purified B lymphocytes although it stimulates the proliferation of T lymphocytes. Lymphoid preparations containing both T and B lymphocytes are more responsive to both mitogens than are either T- or B-lymphocyte preparations. This observation suggested synergy between T and B lymphocytes in the response of unfractionated lymphocytes to mitogens. The basis for this synergy was shown to be the capacity of T lymphocytes to facilitate the proliferation of B lymphocytes cultured with pokeweed mitogen or phytohaemagglutinin. The activity of T lymphocytes is not dependent upon their proliferation or attributable to their release of mitogenic factors. With regard to the clinical evaluation of immune function, our results indicate that the proliferative response of human lymphocytes to phytohaemagglutinin or pokeweed mitogen cannot be directly related to the percentage of T lymphocytes in the lymphoid preparation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. B lymphocytes can be stimulated by concanavalin A in the presence of humoral factors released by T cells. Eur J Immunol. 1972 Feb;2(1):99–101. doi: 10.1002/eji.1830020119. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Elfenbein G. J., Gelfand M. C. Proliferation of mouse bone marrow-derived lymphocytes in vitro: one mechanism of response to concanavalin A and phytochemagglutinin. Cell Immunol. 1975 Jun;17(2):463–476. doi: 10.1016/s0008-8749(75)80050-5. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Rosen F. S., Merler E. Unresponsiveness of human B lymphocytes to phytohaemagglutinin. Nature. 1974 Mar 29;248(447):426–428. doi: 10.1038/248426a0. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Unanue E. R. The immune capacity of lymphocytes after cross-linking of surface immunoglobulin receptors by antibody. J Immunol. 1972 Nov;109(5):1022–1030. [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Rosenstreich D. L., Oppenheim J. J. Induction of lymphokine production by EAC and of blastogenesis by soluble mitogens during human B-cell activation. Nature. 1974 Jun 28;249(460):834–837. doi: 10.1038/249834a0. [DOI] [PubMed] [Google Scholar]
- Mellstedt H. In vitro activation of human T and B lymphocytes by pokeweed mitogen. Clin Exp Immunol. 1975 Jan;19(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Möller G., Andersson J., Sjöberg O. Lipopolysaccharides can convert heterologous red cells into thymus-independent antigens. Cell Immunol. 1972 Aug;4(4):416–424. doi: 10.1016/0008-8749(72)90043-3. [DOI] [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Phillips B., Weisrose E. The mitogenic response of human B lymphocytes to phytohaemagglutinin. Clin Exp Immunol. 1974 Mar;16(3):383–392. [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Vassalli P. Thymus-independent (B) cell proliferation in spleen cell cultures of mouse radiation chimeras stimulated by phytohemagglutinin or allogeneic cells. J Exp Med. 1972 Oct 1;136(4):962–967. doi: 10.1084/jem.136.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Weksler M. E., Kuntz M. E., Lockshin M., Kohn R., Eisenhauer A. C. Letter: Lymphocyte response. Lancet. 1974 Jun 1;1(7866):1116–1116. doi: 10.1016/s0140-6736(74)90600-x. [DOI] [PubMed] [Google Scholar]
- Winkelstein A. Augmentation of PHA responsiveness in mixed thymus-marrow cultures. J Immunol. 1971 Jul;107(1):195–200. [PubMed] [Google Scholar]


