Abstract
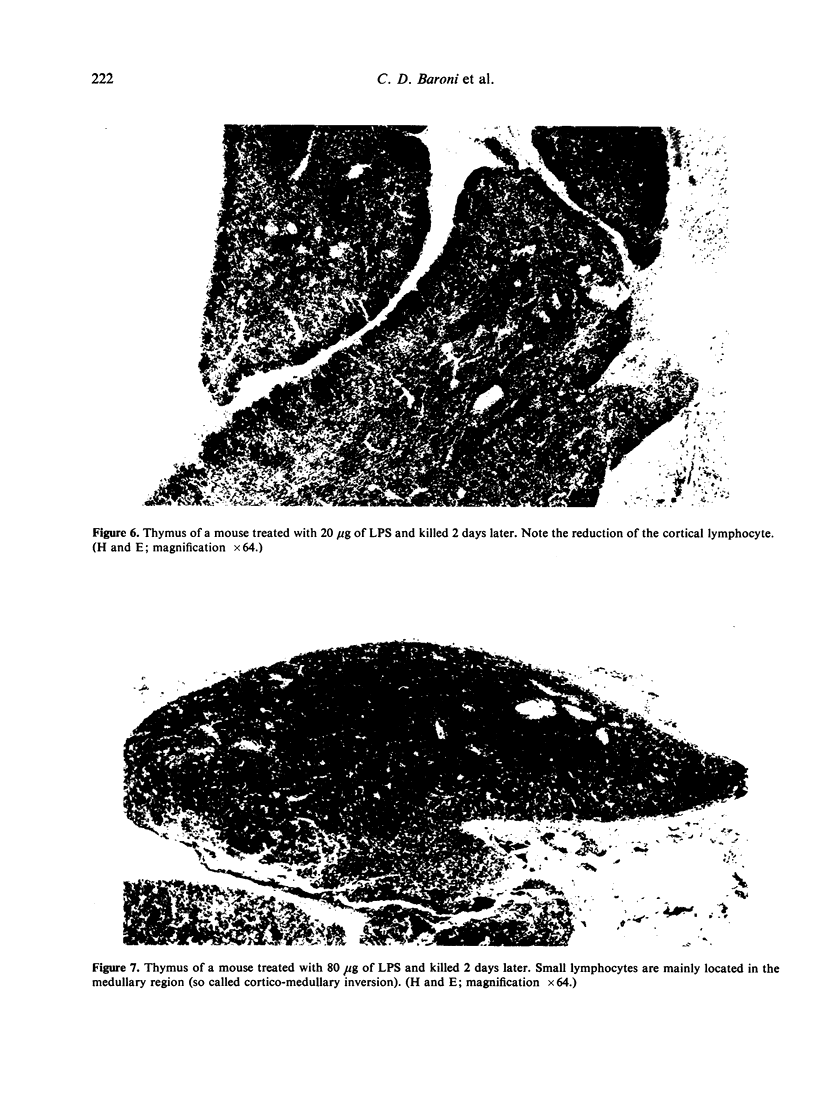
In the present study we have investigated the biological effects on thymus lymphocytes resulting from Escherichia coli lipopolysaccharide (LPS) treatment in young adult mice. It has been established that LPS induces the following effects: (a) a dose-dependent reduction of thymus weight contemporaneous with a rise in the anti-LPS antibody response; (b) an increase of killer activity of thymus cells; (c) an enhancement of thymocytes helper activity; (d) a reduction of theta-positive cells in the thymus; (e) a cellular depletion in the thymus cortex. These data, indicating that LPS selects in the thymus a population of cells more efficient in expressing both killer and helper functions, are interpreted as caused by an increased rate of cortisol secretion induced by the LPS treatment.
Full text
PDF

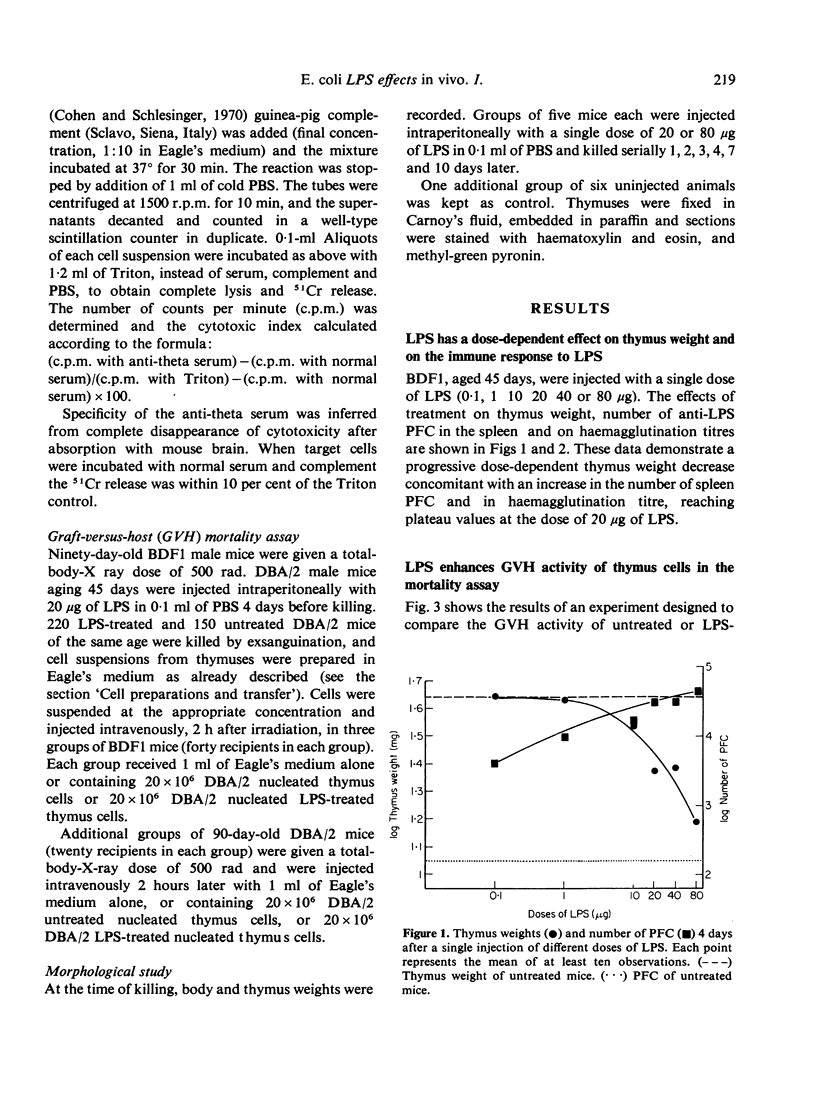
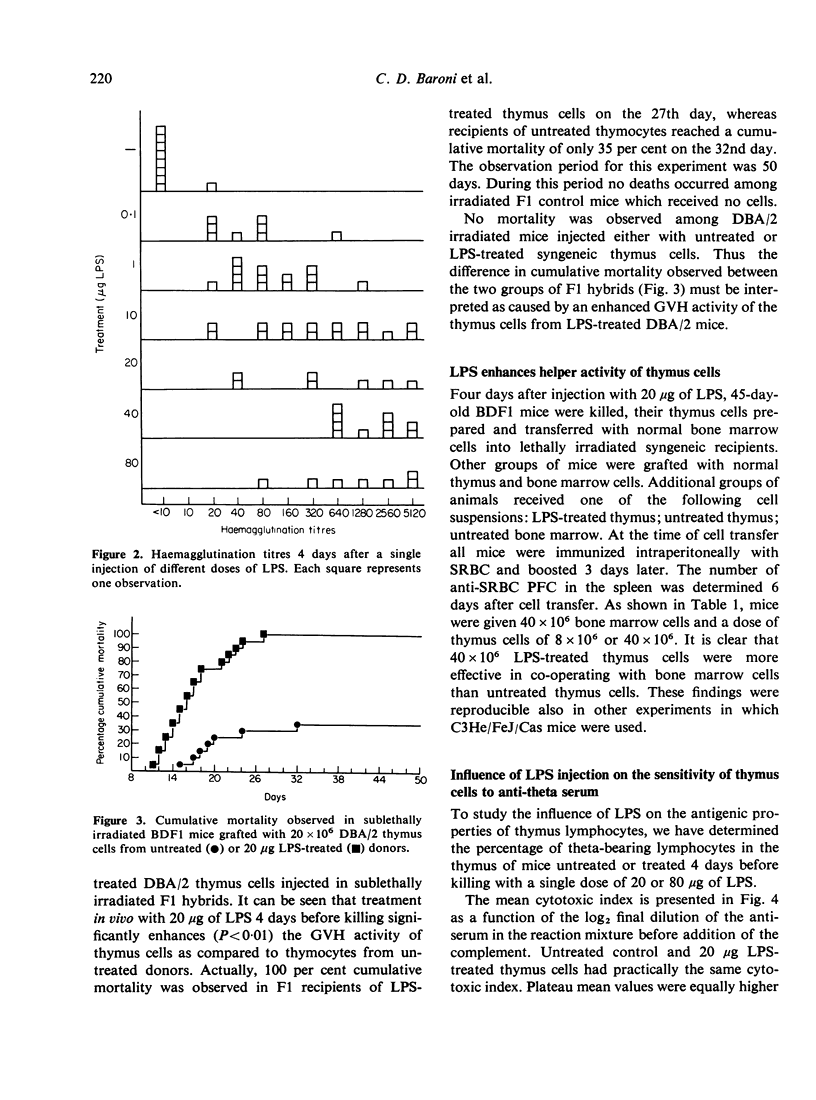
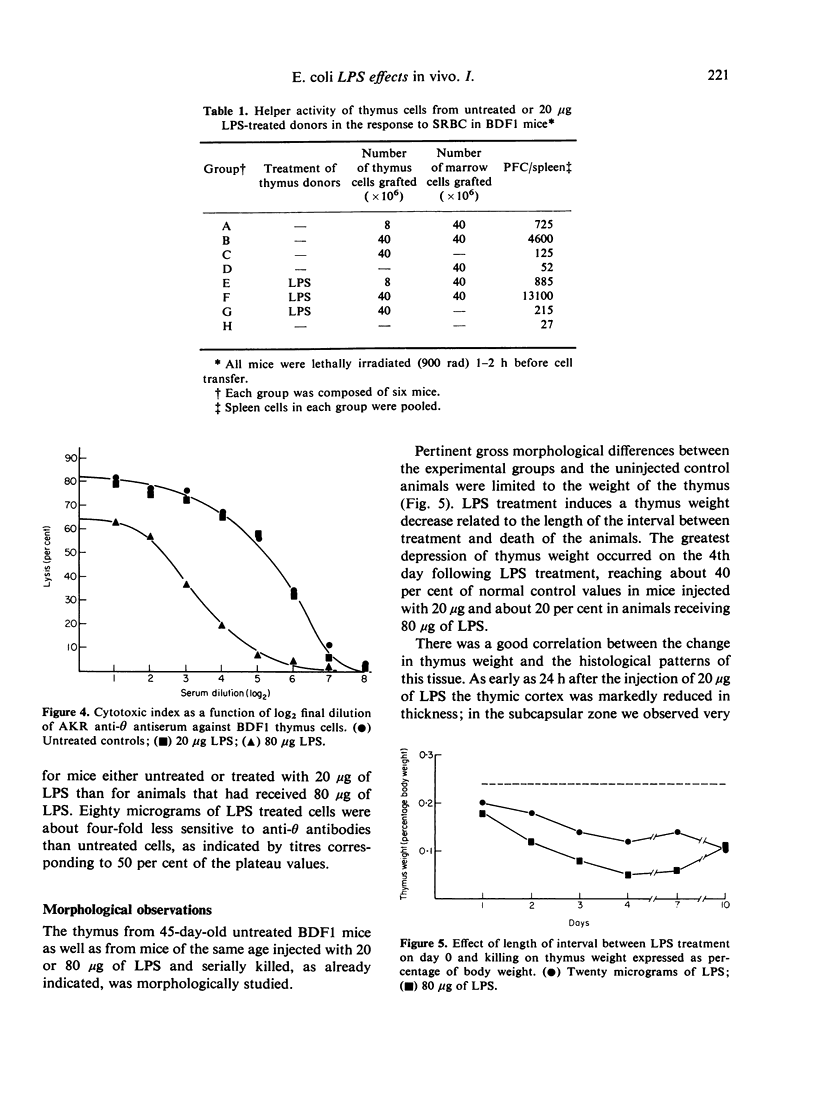


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AL-ASKARI S., ZWEIMAN B., LAWRENCE H. S., THOMAS L. THE EFFECT OF ENDOTOXIN ON SKIN HOMOGRAFTS IN RABBITS. J Immunol. 1964 Nov;93:742–748. [PubMed] [Google Scholar]
- Adorini L., Ruco L., Uccini S., De Franceschi G. S., Baroni C. D., Doria G. Biological effects of Escherichia coli lipopolysaccharide (LPS) in vivo. II. Selection in the mouse thymus of PHA- and con A-responsive cells. Immunology. 1976 Aug;31(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield P., Christie G. H., Howard J. G. Alternative potentiating and inhibitory effects of GVH reaction on formation of antibodies against a thymus-independent polysaccharide (S3). J Immunol. 1973 Jul;111(1):72–84. [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G., Agarossi G. Thymus dependence of adoptive immunity in irradiated mice. Transplantation. 1968 Mar;6(2):218–229. doi: 10.1097/00007890-196803000-00008. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Billings P., Cohn M. Functional characteristics of Peyer's patch lymphoid cells. II. Lipopolysaccharide is thymus dependent. J Exp Med. 1974 Feb 1;139(2):407–413. doi: 10.1084/jem.139.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. Effects of bacterial lipopolysaccharide on the induction and expression of cell-mediated immunity. II. Stimulation of the efferent arc. J Immunol. 1975 Jan;114(1 Pt 2):447–451. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E., Pardon P. Effects of bacterial lipopolysaccharide on the induction and expression of cell-mediated immunity. I. Depression of the afferent arc. J Immunol. 1975 Jan;114(1 Pt 2):442–446. [PubMed] [Google Scholar]
- Möller G. 19S antibody production against soluble lipopolysaccharide antigens by individual lymphoid cells in vitro. Nature. 1965 Sep 11;207(5002):1166–1168. doi: 10.1038/2071166a0. [DOI] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Skopińska E. Some effects of Escherichia coli endotoxin on the graft-versus-host reaction in mice. Transplantation. 1972 Oct;14(4):432–437. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Veit B. C., Michael J. G. The lack of thymic influence in regulating the immune response to Escherichia coli 0127 endotoxin. J Immunol. 1972 Sep;109(3):547–553. [PubMed] [Google Scholar]
- Westphal O. Bacterial endotoxins. The second Carl Prausnitz Memorial Lecture. Int Arch Allergy Appl Immunol. 1975;49(1-2):1–43. [PubMed] [Google Scholar]