Abstract
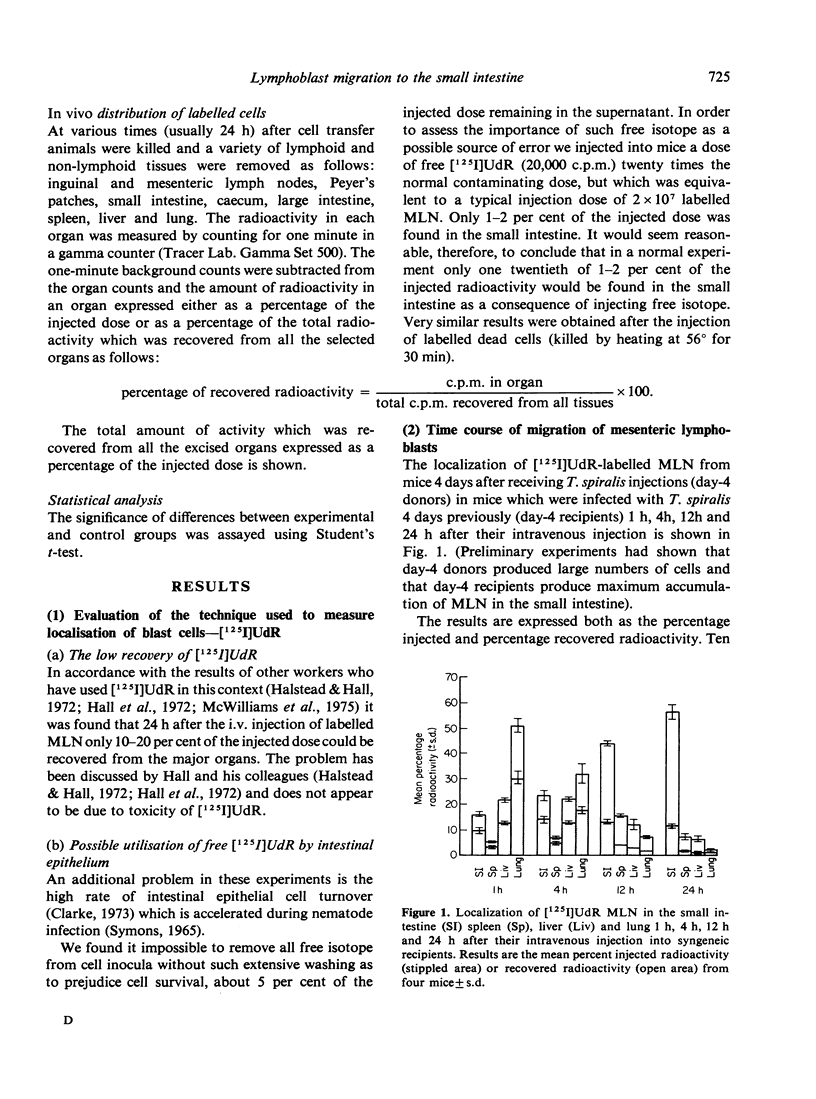
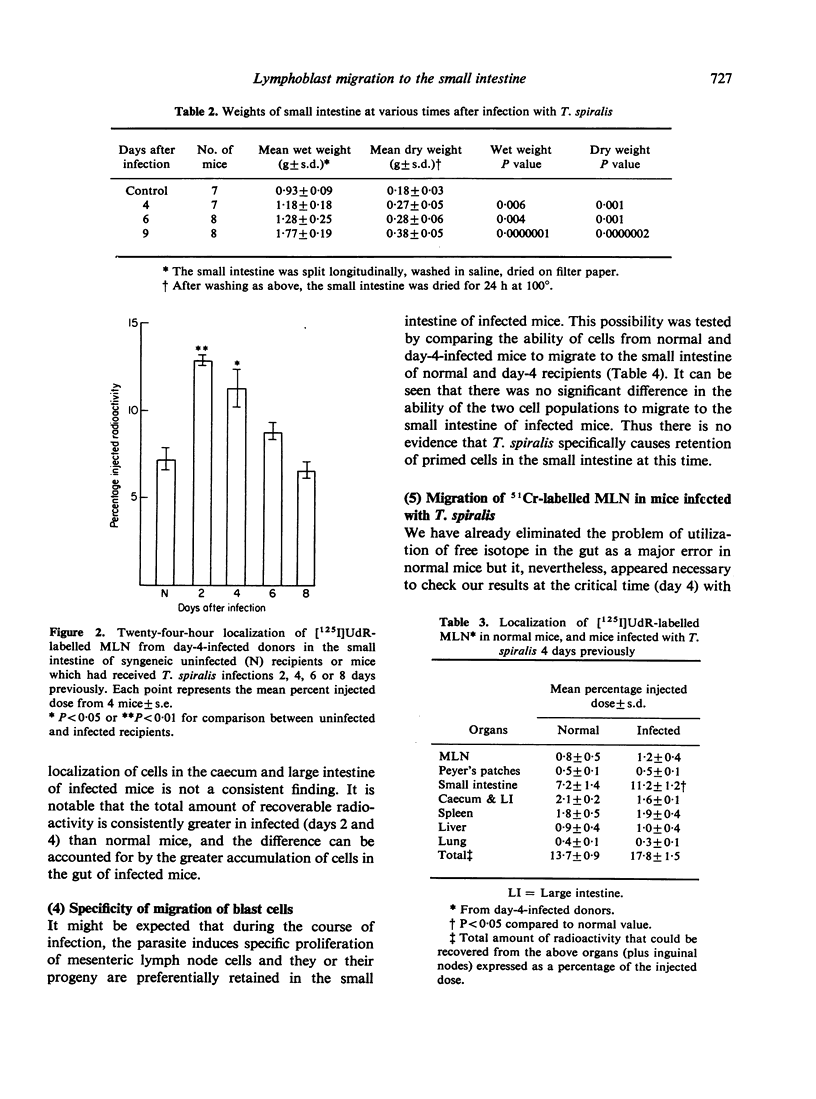
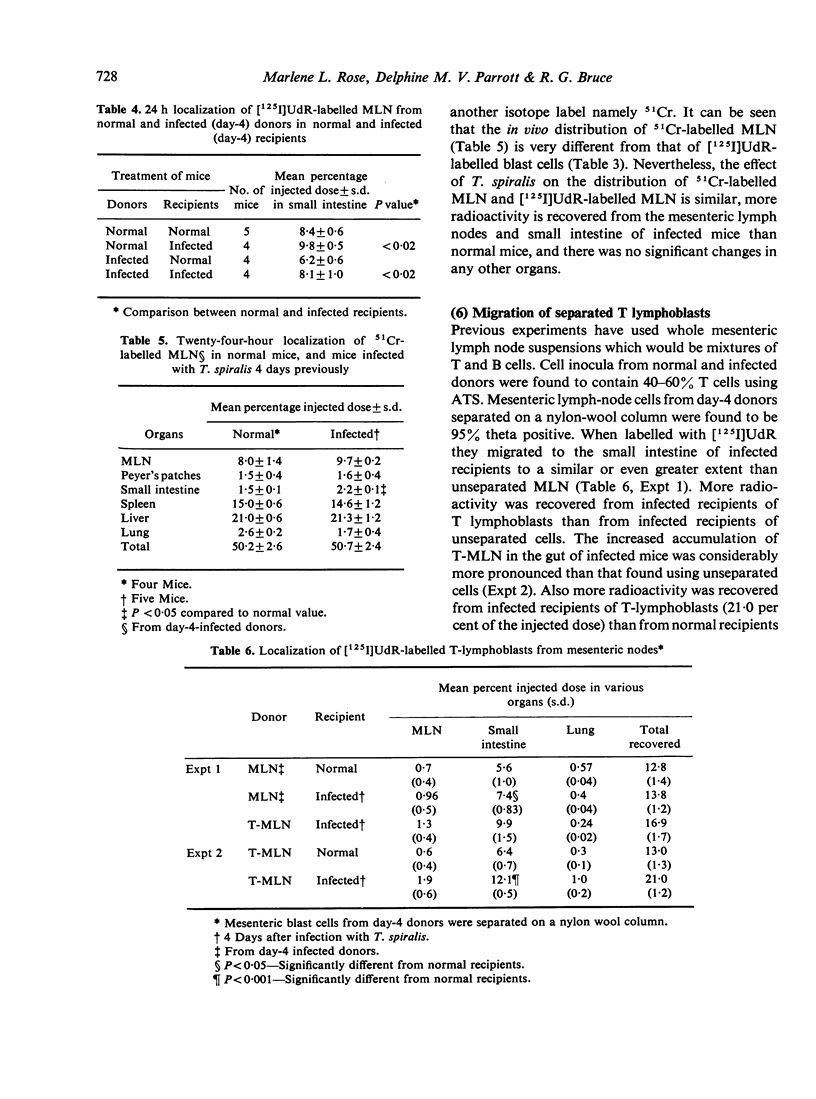
The migration of [125I]UdR-labelled mesenteric lymph node cells in NIH strain mice at various times after inis produced an enhanced accumulation of mesenteric immunoblasts in the small intestine at 2 and 4 days after infection but not at later times. The enhanced migration occurred when using cells from both uninfected and infected donors, denoting an absence of antigenic specificity. This effect is not secondary to a reduced arrival of cells at sites away from the gut in infected mice, but to a primary increase of the arrival in the small intestine. Mesenteric T lymphoblasts (separated on a nylon-wool column) migrated to the small intestine of uninfected recipients and appear to be a major portion of the population which migrate to the gut of infected recipients. Our results were confirmed using 51Cr to label mesenteric cells. We conclude that the parasite causes the small intestine to become more attractive or retentive for mesenteric blast cells early during infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke R. M. Progress in measuring epithelial turnover in the villus of the small intestine. Digestion. 1973;8(2):161–175. doi: 10.1159/000197311. [DOI] [PubMed] [Google Scholar]
- Delorme E. J., Hodgett J., Hall J. G., Alexander P. The cellular immune response to primary sarcomata in rats. I. The significance of large basophilic cells in the thoracic duct lymph following antigenic challenge. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):229–236. doi: 10.1098/rspb.1969.0089. [DOI] [PubMed] [Google Scholar]
- Dineen J. K., Ogilvie B. M., Kelly J. D. Expulsion of Nippostrongylus brasiliensis from the intestine of rats. Collaboration between humoral and cellular components of the immune response. Immunology. 1973 Mar;24(3):467–475. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Griscelli C., Vassalli P., McCluskey R. T. The distribution of large dividing lymph node cells in syngeneic recipient rats after intravenous injection. J Exp Med. 1969 Dec 1;130(6):1427–1451. doi: 10.1084/jem.130.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Parry D. M., Smith M. E. The distribution and differentiation of lymph-borne immunoblasts after intravenous injection into syngeneic recipients. Cell Tissue Kinet. 1972 May;5(3):269–281. doi: 10.1111/j.1365-2184.1972.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Halstead T. E., Hall J. G. The homing of lymph-borne immunoblasts to the small gut of neonatal rats. Transplantation. 1972 Sep;14(3):339–346. doi: 10.1097/00007890-197209000-00009. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R. Protective immunity to Nippostrongylus brasiliensis in the rat. Central role of the lymphocyte in worm expulsion. Immunology. 1972 May;22(5):767–773. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Dineen J. K. The cellular transfer to immunity to Nippostrongylus brasiliensis in inbred rats (Lewis strain). Immunology. 1972 Feb;22(2):199–210. [PMC free article] [PubMed] [Google Scholar]
- Larsh J. E., Jr, Weatherly N. F. Cell-mediated immunity against certain parasitic worms. Adv Parasitol. 1975;13:183–222. doi: 10.1016/s0065-308x(08)60321-8. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Characteristics of mesenteric lymph node cells homing to gut-associated lymphoid tissue in syngeneic mice. J Immunol. 1975 Jul;115(1):54–58. [PubMed] [Google Scholar]
- Ogilvie B. M., Love R. J. Co-operation between antibodies and cells in immunity to a nematode parasite. Transplant Rev. 1974;19(0):147–169. doi: 10.1111/j.1600-065x.1974.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- SYMONS L. E. KINETICS OF THE EPITHELIAL CELLS, AND MORPHOLOGY OF VILLI AND CRYPTS IN THE JEJUNUM OF THE RAT INFECTED BY THE NEMATODE NIPPOSTRONGYLUS BRASILIENSIS. Gastroenterology. 1965 Aug;49:158–168. [PubMed] [Google Scholar]
- Walls R. S., Carter R. L., Leuchars E., Davies A. J. The immunopathology of trichiniasis in T-cell deficient mice. Clin Exp Immunol. 1973 Feb;13(2):231–242. [PMC free article] [PubMed] [Google Scholar]


