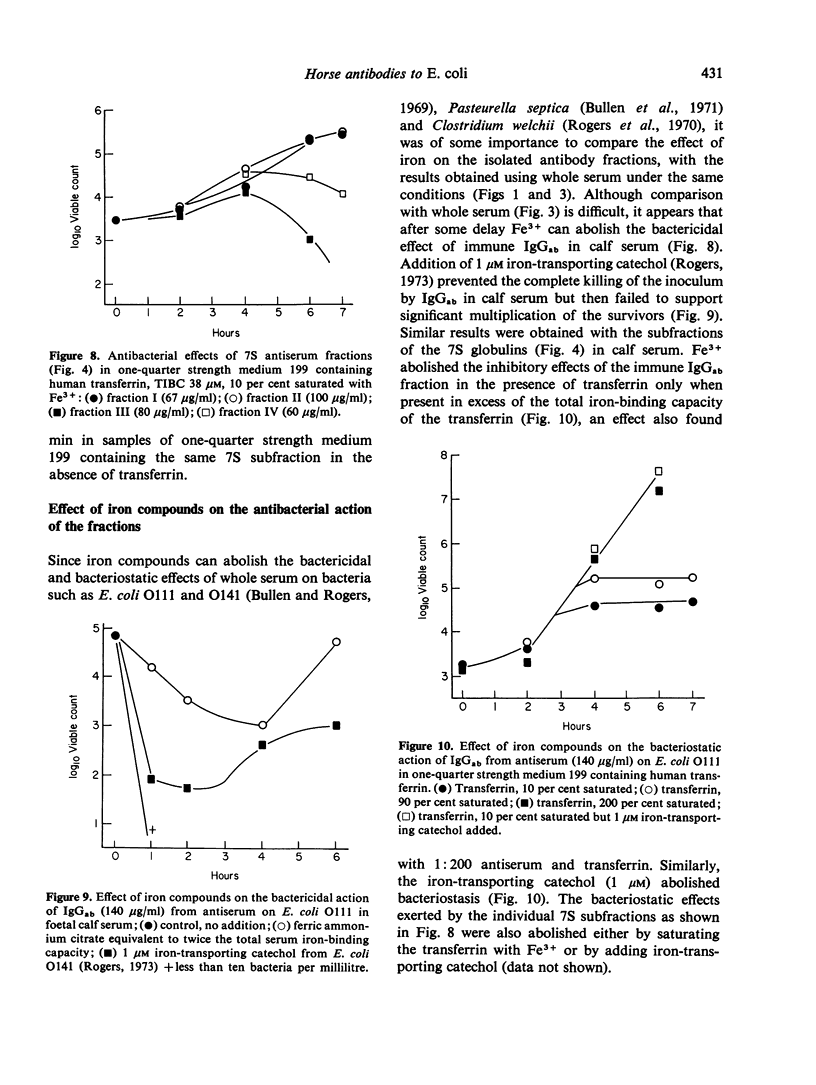
Abstract
Previous work showed that the virulence of Escherichia coli O141 was related to its ability to secrete catechols capable of transporting iron from serum transferrin to the bacterial cell. E. coli O111 also produced similar compounds in synthetic medium but was unable to do so in serum. It was postulated that antibody interfered with the production of these substances by this strain. The present experiments show that horse antiserum to E. coli O111 can induce bacterial killing in foetal calf serum, which, in contrast to the bactericidal effect of normal rabbit serum, cannot be reversed by Fe3+. Five IgG subfractions of increasing electrophoretic mobility were isolated from the 7S fraction of the antiserum, all of which exerted a bactericidal effect on E. coli O111 in calf serum which could not be prevented by Fe3+. The bacteriostatic effect induced by the fractions in the presence of transferrin could, however, be reversed by Fe3+. IgG and IgT were isolated from normal serum but neither of these activated calf serum complement or induced bacteriostasis in the presence of transferrin. These results show that specific antibody is responsible for these antibacterial effects and point to new problems concerning the role of iron compounds in the antibacterial effects of normal and specific immune serum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta O., Barta V., Ingram D. G., Hubbert W. T. Bactericidal activity of bovine fetal serum against smooth and rough strains of Escherichia coli. Am J Vet Res. 1972 Apr;33(4):731–740. [PubMed] [Google Scholar]
- Barta O., Barta V., Ingram D. G. Postnatal development of bactericidal activity in serum from conventional and colostrum-deprived calves. Am J Vet Res. 1972 Apr;33(4):741–750. [PubMed] [Google Scholar]
- Bullen J. J., Leigh L. C., Rogers H. J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology. 1968 Oct;15(4):581–588. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J. Bacterial iron metabolism and immunity to Pasteurella septica and Escherichia coli. Nature. 1969 Oct 25;224(5217):380–382. doi: 10.1038/224380a0. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Wilson A. B., Cushnie G. H., Rogers H. J. The abolition of the protective effect of Pasteurella septica antiserum by iron compounds. Immunology. 1968 Jun;14(6):889–898. [PMC free article] [PubMed] [Google Scholar]
- Fletcher J., Goldstein E. The effect of parenteral iron preparations on experimental pyelonephritis. Br J Exp Pathol. 1970 Jun;51(3):280–285. [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. Abnormal phenylalanyl-tRNA found in serum inhibited Escherichia coli, strain 0111. FEBS Lett. 1972 Sep 1;25(1):159–164. doi: 10.1016/0014-5793(72)80476-9. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Mechanism of action of specific antiserum on Pasteurella septica. Selective inhibition of net macromolecular synthesis and its reversal by iron compounds. Eur J Biochem. 1971 Nov 11;23(1):69–76. doi: 10.1111/j.1432-1033.1971.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Helms C. M., Allen P. Z. Studies on equine immunoglobulins. II. Anigenic interrelationships among horse IgG(T) and antipneumococcal gamma-1-component. J Immunol. 1970 Nov;105(5):1253–1263. [PubMed] [Google Scholar]
- Kim Y. D., Karush F. Equine anti-hapten antibody. VII. Anti-lactoside antibody induced by a bacterial vaccine. Immunochemistry. 1973 Jun;10(6):365–371. doi: 10.1016/0019-2791(73)90087-6. [DOI] [PubMed] [Google Scholar]
- Muschel L. H., Gustafson L., Larsen L. J. Re-examination of the Neisser-Wechsberg (antibody prozone) phenomenon. Immunology. 1969 Oct;17(4):525–533. [PMC free article] [PubMed] [Google Scholar]
- ROCKEY J. H., KLINMAN N. R., KARUSH F. EQUINE ANTIHAPTEN ANTIBODY. I. 7S BETA-2A- AND 1OS GAMMA-1- GLOBULIN COMPONENTS OF PURIFIED ANTI-BETA-LACTOSIDE ANTIBODY. J Exp Med. 1964 Oct 1;120:589–609. doi: 10.1084/jem.120.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerman J. P., Querinjean P., Heremans J. F. Studies on the IgA system of the horse. Immunology. 1971 Sep;21(3):443–454. [PMC free article] [PubMed] [Google Scholar]