Abstract
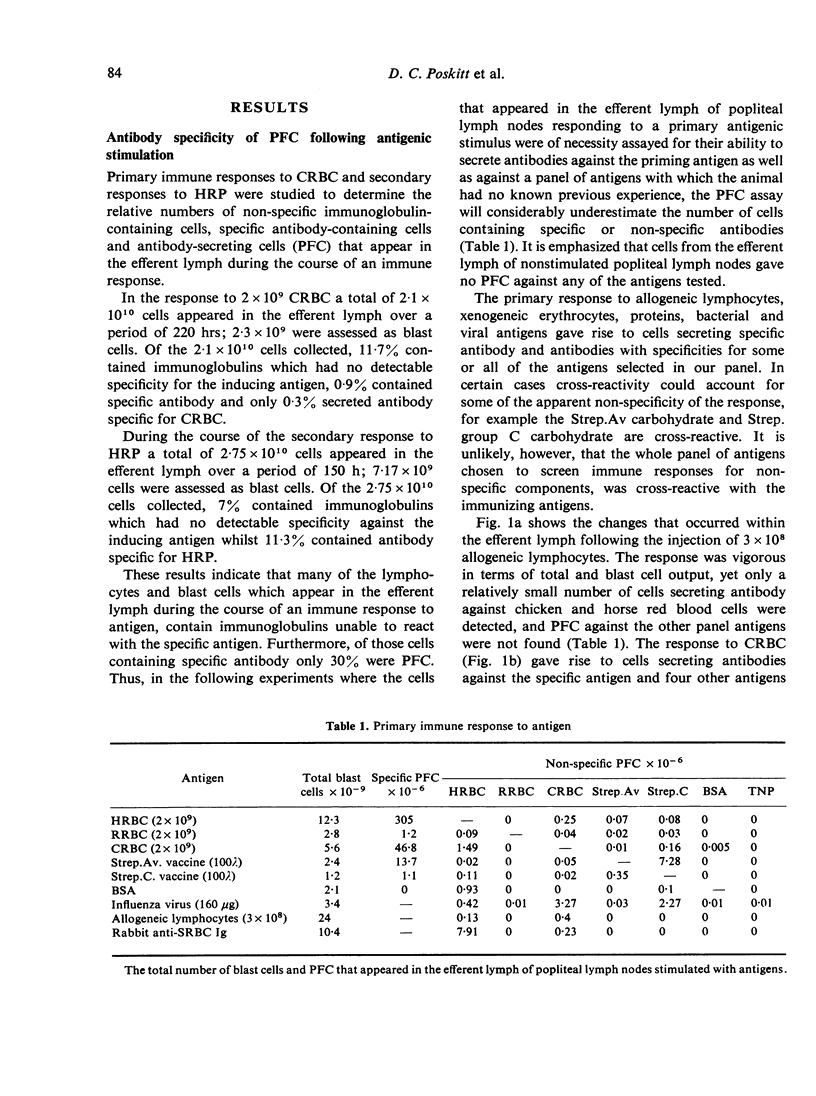
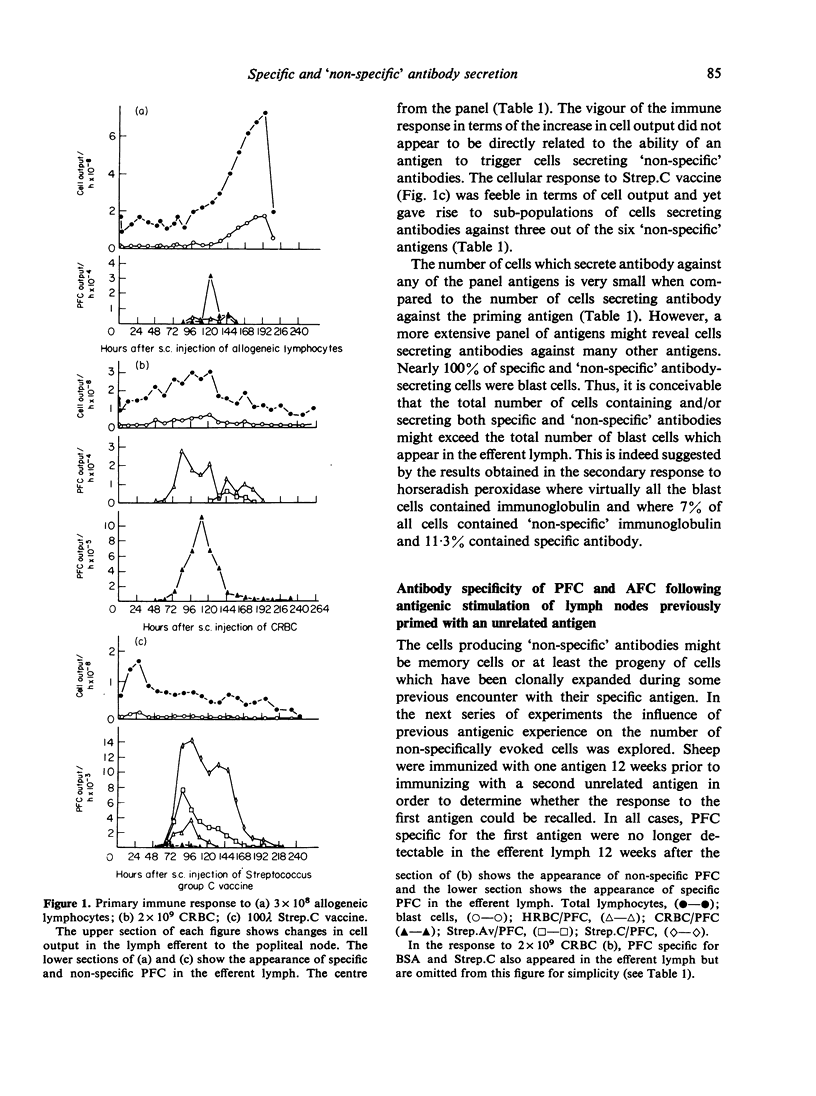
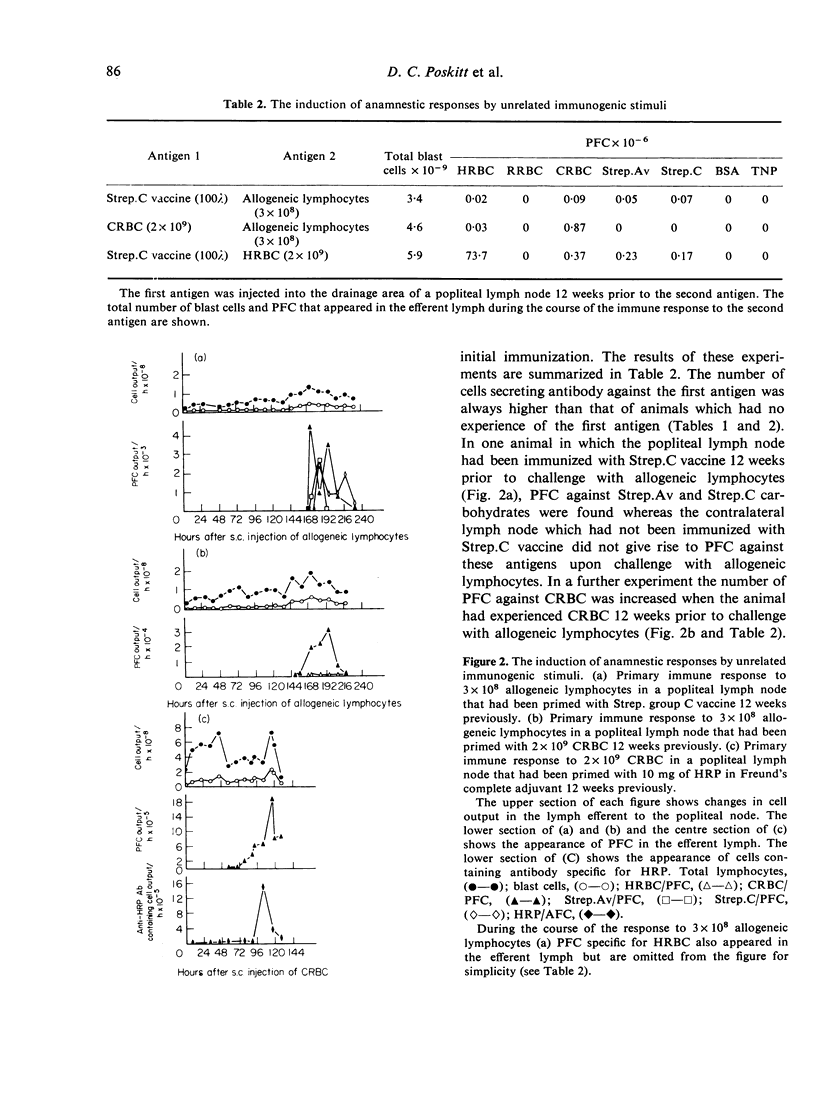
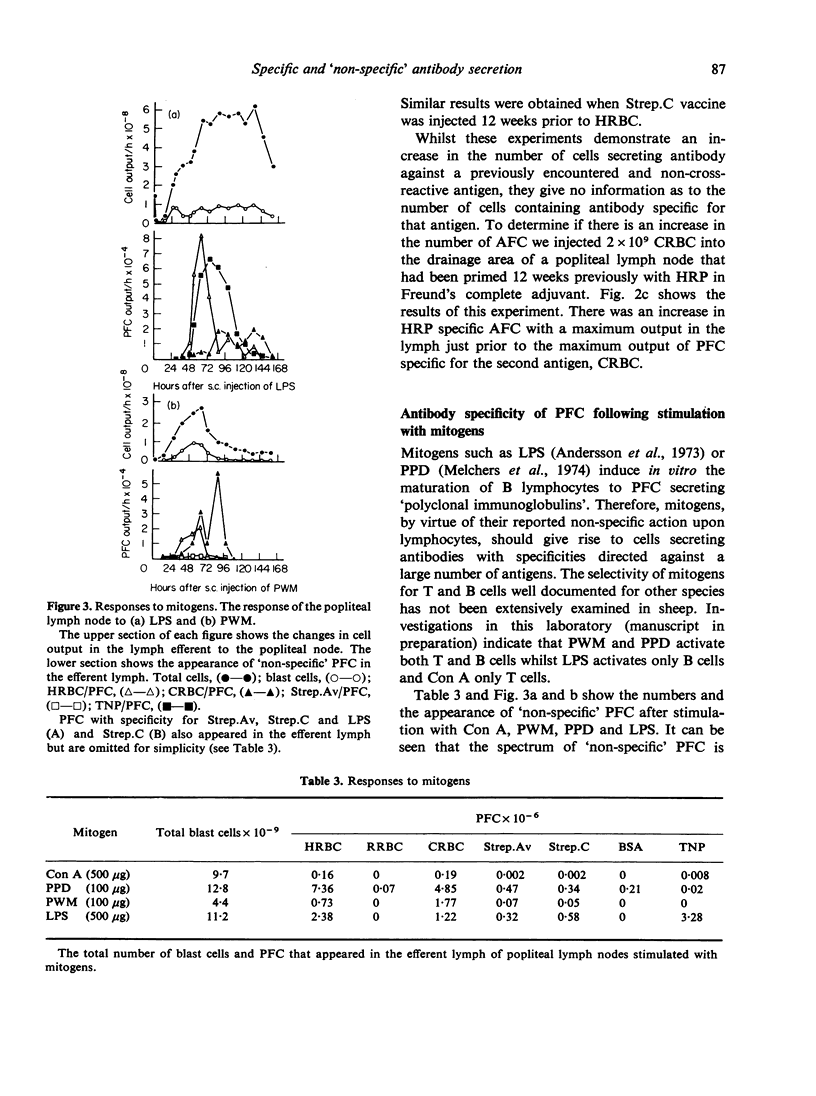
Immunization of single lymph nodes with various antigens led to the appearance of cells in the efferent lymph that secreted antibody specific for the antigen which induced their formation and for a number of unrelated, non-crossreacting antigens. Immunization of single lymph nodes with mitogens led to the appearance of cells secreting antibodies specific for an even greater number of antigens, including one (TNP) that in all probability is not present in the animals' natural environment. When the node was primed with one antigen, a subsequent challenge with an unrelated antigen 12 weeks later led to the appearance of greater numbers of cells containing and secreting antibody against the previously experienced antigen, than was the case in unprimed lymph nodes. These findings indicate that the immune response to antigen provokes the maturation of lymphocytes of specificities unrelated to that of the injected immunogen. Such a mechanism may be important in maintaining immunological memory. Mitogens may directly activate lymphocytes into maturation and expression as antibody-secreting cells, whereas antigens appear to act indirectly.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S. Correlations between immunoglobulin- and antibody-synthesizing cells during primary and secondary immune responses of rats immunized with peroxidase. Immunology. 1976 Apr;30(4):537–547. [PMC free article] [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S. Etude de la formation des anticorps anti-tyrosinase au cours de la réponse immunitaire chez le lapin. I. Etude cellulaire. Ann Immunol (Paris) 1973 Feb;124(1):121–131. [PubMed] [Google Scholar]
- Avrameas S. Immunoenzyme techniques: enzymes as markers for the localization of antigens and antibodies. Int Rev Cytol. 1970;27:349–385. doi: 10.1016/s0074-7696(08)61250-4. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Lespinats G. Détection d'anticorps dans des cellules immunocompétentes d'animaux immunisés avec des enzymes. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 17;265(3):302–304. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- De Vos-Cloetens C., Minsart-Baleriaux V., Urbain-Vansanten G. Possible relationships between antibodies and non-specific immunoglobulins simultaneously induced after antigenic stimulation. Immunology. 1971 Jun;20(6):955–962. [PMC free article] [PubMed] [Google Scholar]
- FRANCIS T., Jr Influenza: the new acquayantance. Ann Intern Med. 1953 Aug;39(2):203–221. doi: 10.7326/0003-4819-39-2-203. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966 Sep 1;124(3):347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost H., Braun D. G., Poskitt D., Cahill R. N., Trnka Z. Antipolysaccharide antibodies of restricted heterogeneity secreted by a single lymph node. J Exp Med. 1976 Mar 1;143(3):707–711. doi: 10.1084/jem.143.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Selective triggering of B cell subpopulations by mitogens. Eur J Immunol. 1974 Nov;4(11):771–776. doi: 10.1002/eji.1830041113. [DOI] [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Stewart D. C. Potentiation of rat reaginic (IgE) antibody by helminth infection. Simultaneous potentiation of separate reagins. Immunology. 1972 Nov;23(5):749–755. [PMC free article] [PubMed] [Google Scholar]
- Loor F. On the existence of heterospecific antibodies in sera from rabbits immunized against tobacco mosaic virus determinants. Immunology. 1971 Oct;21(4):557–564. [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. The kinectics of proliferation and maturation of mitogen-activated bone marrow-derived lymphocytes. Eur J Immunol. 1974 Oct;4(10):687–691. doi: 10.1002/eji.1830041010. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Avrameas S., Ternynck T. Intracellular distribution of antibody in immunocytes responding to primary challenge with horseradish peroxidase. Am J Pathol. 1973 May;71(2):239–260. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Ternynck T., Avrameas S. A comparison of specific antibody and immunoglobulin synthesising immunocytes in peroxidase stimulated lymph nodes. Ann Immunol (Paris) 1974 Jan;125C(1-2):231–238. [PubMed] [Google Scholar]
- Miller H. R., Ternynck T., Avrameas S. Synthesis of antibody and immunoglobulins without detectable antibody function in cells responding to horseradish peroxidase. J Immunol. 1975 Feb;114(2 Pt 1):626–629. [PubMed] [Google Scholar]
- Moticka E. J. Cellular basis and nature of the polyclonal hyperimmunoglobulinemia induced by antigenic challenge. Cell Immunol. 1975 Sep;19(1):32–40. doi: 10.1016/0008-8749(75)90289-0. [DOI] [PubMed] [Google Scholar]
- Moticka E. J. The non-specific stimulation of immunoglobulin secretion following specific stimulation of the immune system. Immunology. 1974 Sep;27(3):401–412. [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Pachmann K., Killander D., Wigzell H. Increase in intracellular immunoglobulins in the majority of splenic lymphoid cells after primary immunization. Eur J Immunol. 1974 Feb;4(2):138–145. doi: 10.1002/eji.1830040214. [DOI] [PubMed] [Google Scholar]
- Read S. E., Braun D. G. In vitro antibody response of primed rabbit peripheral blood lymphocytes to group A variant streptococcal polysaccharide. Eur J Immunol. 1974 Jun;4(6):422–426. doi: 10.1002/eji.1830040607. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Shimada K., Silverstein A. M. Induction of booster antibody formation without specific antigenic drive. Cell Immunol. 1975 Aug;18(2):484–488. doi: 10.1016/0008-8749(75)90075-1. [DOI] [PubMed] [Google Scholar]
- Sweet G. H., Welborn F. L. Use of chromium chloride as the coupling agent in a modified plaque assay. Cells producing anti-protein antibody. J Immunol. 1971 May;106(5):1407–1410. [PubMed] [Google Scholar]
- Urbain-Vansanten G. Concomitant synthesis, in separate cells, of non-reactive immunoglobulins and specific antibodies after immunization with tobacco mosaic virus. Immunology. 1970 Nov;19(5):783–797. [PMC free article] [PubMed] [Google Scholar]


