Abstract
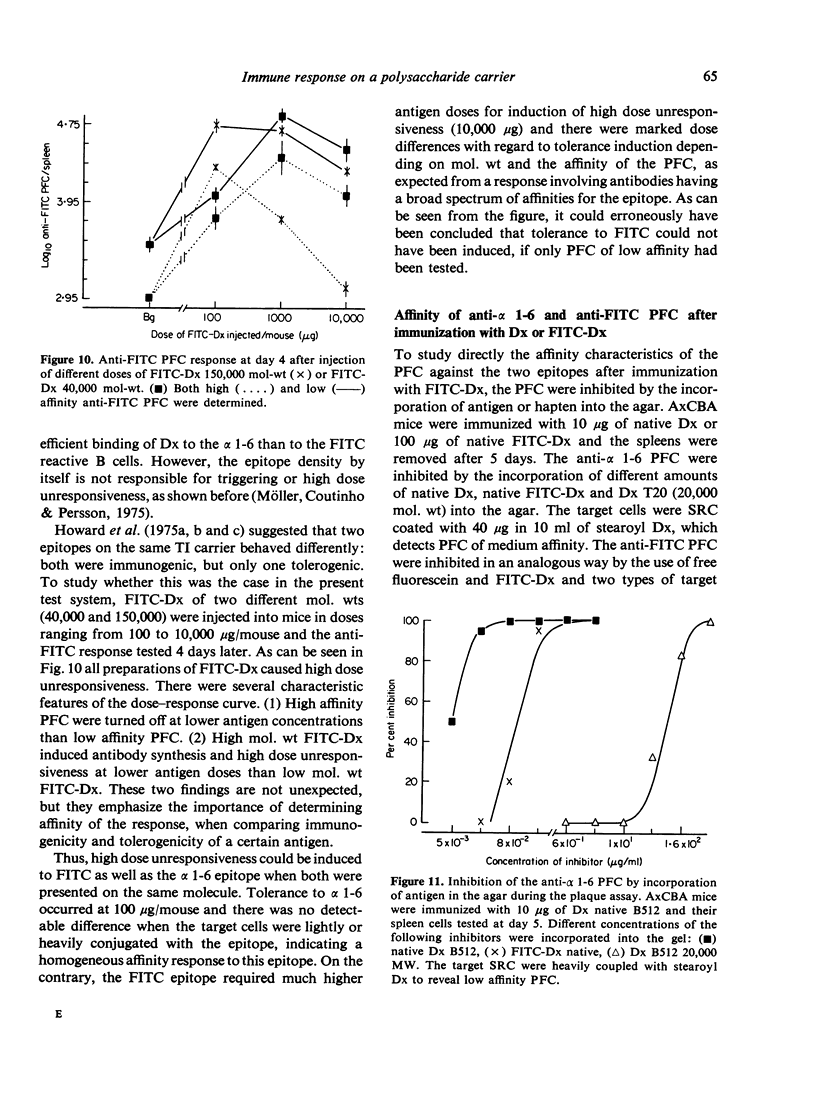
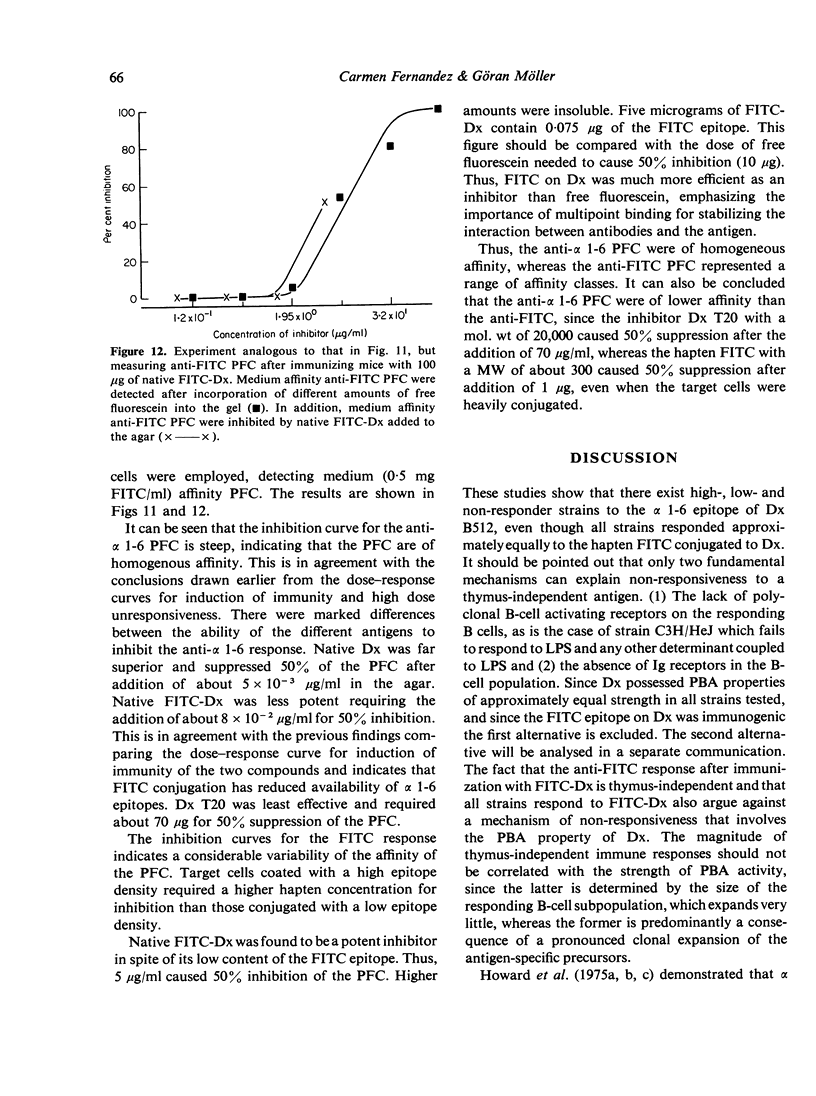
Immunogenicity and tolerogenicity of two epitopes (alpha 1-6 and FITC) on the same dextran B 512 carrier were investigated. The following conclusions were made: (1) Both epitopes were thymus-independent and immunogenic and tolerogenic as well. (2) The marked dose differences between the two epitopes with regard to tolerance induction were found to be a consequence of the affinity and the heterogeneity of the responding B cells as well as the epitope density employed for detecting the PFC in a predictable way. (3) The alpha 1-6 response, in contrast to the anti-FITC response, was homogeneous and of low affinity and the number of precursor B cells was low. (4) Different mouse strains were found to be high-, low- or non-responders to alpha 1-6, but all the strains tested responded to the FITC epitope coupled to dextran. (5) Dextran and FITC-dextran were polyclonal B cell activators in the strains tested, irrespective of their ability to respond to the alpha 1-6 epitope. The findings indicate that epitope density and mol. wt of the immunogen as well as Ig receptor affinity for the epitope on the B cells are variables which markedly influence the binding of the immunogen to the specific B cells and therefore affect the delivery of the non-specific triggering signal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg B., Geckeler W. R., Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972 Jul 14;177(4044):178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- Bullock W. W., Möller E. "Spontaneous" B cell activation due to loss of normal mouse serum suppressor. Eur J Immunol. 1972 Dec;2(6):514–517. doi: 10.1002/eji.1830020609. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Bullock W. W., Möller G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J Exp Med. 1974 Jan 1;139(1):74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Editorial: Immune activation of B cells: evidence for 'one nonspecific triggering signal' not delivered by the Ig receptors. Scand J Immunol. 1974;3(2):133–146. [PubMed] [Google Scholar]
- Coutinho A., Möller G. In vitro induction of specific immune responses in the absence of serum: requirement for nonspecific T or B cell mitogens. Eur J Immunol. 1973 Sep;3(9):531–537. doi: 10.1002/eji.1830030902. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Richter W. Molecular basis of B-cell activation. I. Mitogenicity of native and substituted dextrans. Scand J Immunol. 1974;3(3):321–328. doi: 10.1111/j.1365-3083.1974.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Courtenay B. M. Influence of molecular structure on the tolerogenicity of bacterial dextrans. II. The alpha1--3-linked epitope of dextran B1355. Immunology. 1975 Oct;29(4):599–610. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Courtenay B. M., Vicari G. Influence of molecular structure of the tolerogenicity of bacterial dextrans. III. Dissociation between tolerance and immunity to the alpha1--6- and alpha1--3-linked epitopes of dextran B1355. Immunology. 1975 Oct;29(4):611–619. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Vicari G., Courtenay B. M. Influence of molecular structure on the tolerogenicity of bacterial dextrans. I. The alpha1--6-linked epitope of dextran B512. Immunology. 1975 Oct;29(4):585–597. [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Effect of B-cell mitogens on lymphocyte subpopulations possesing C'3 and Fc receptors. J Exp Med. 1974 Apr 1;139(4):969–982. doi: 10.1084/jem.139.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen V. J., Mäkelä O. Effect of the number of haptens coupled to each erythrocyte on haemolytic plaque formation. Immunology. 1969 Mar;16(3):399–407. [PMC free article] [PubMed] [Google Scholar]


