Abstract
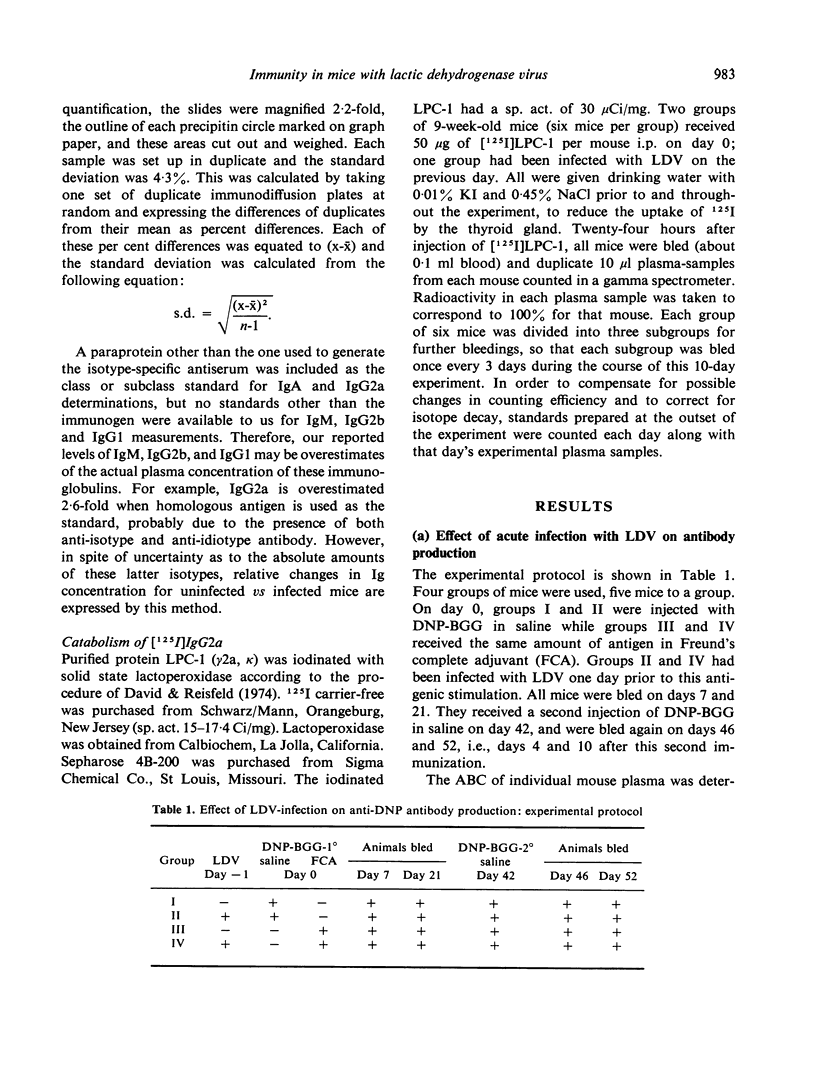
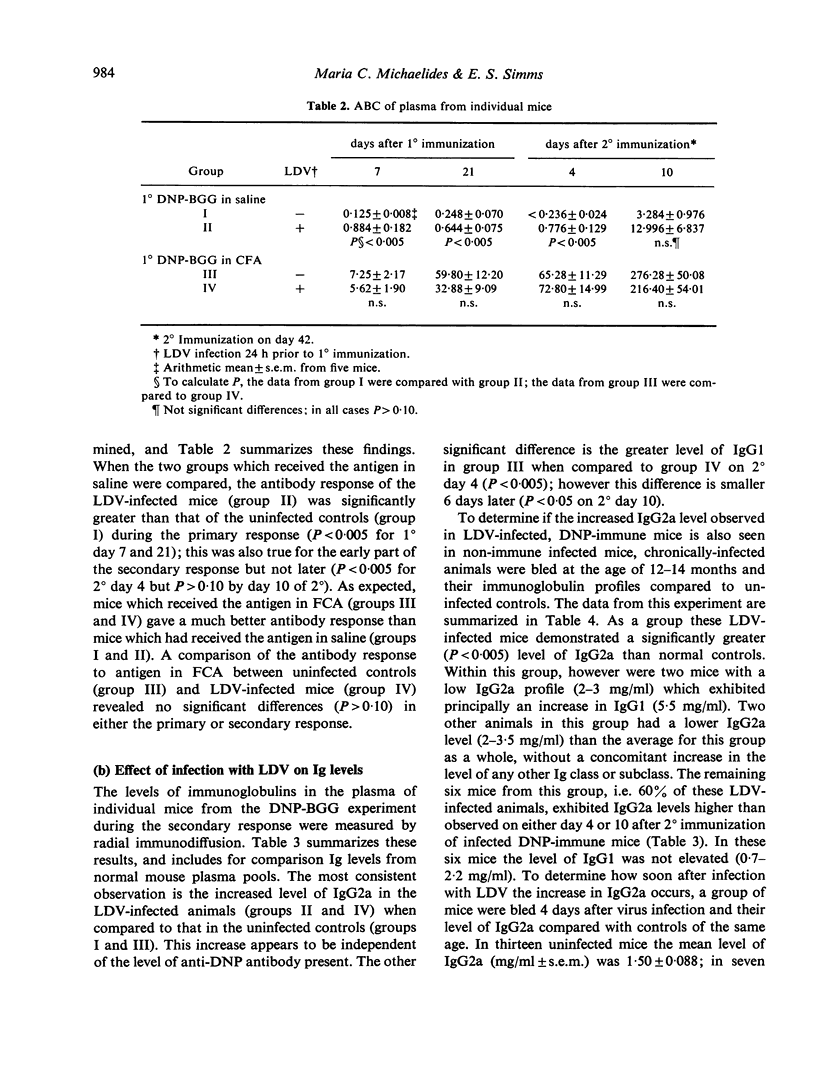
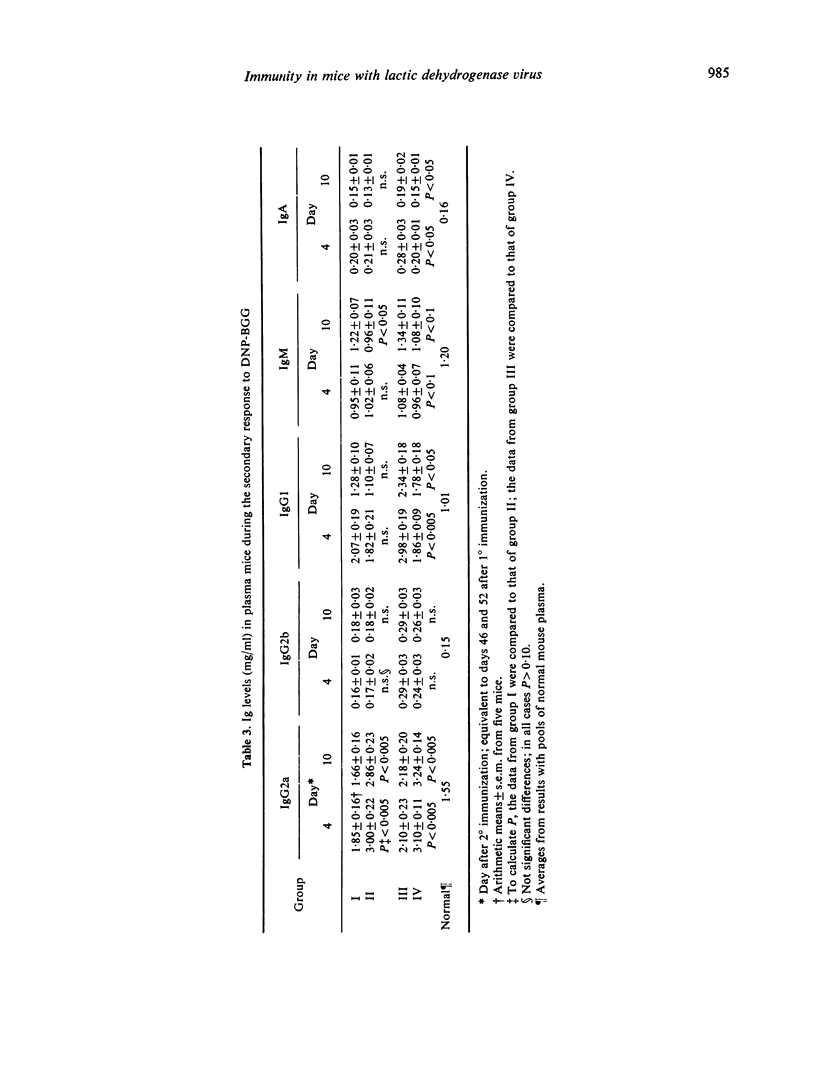
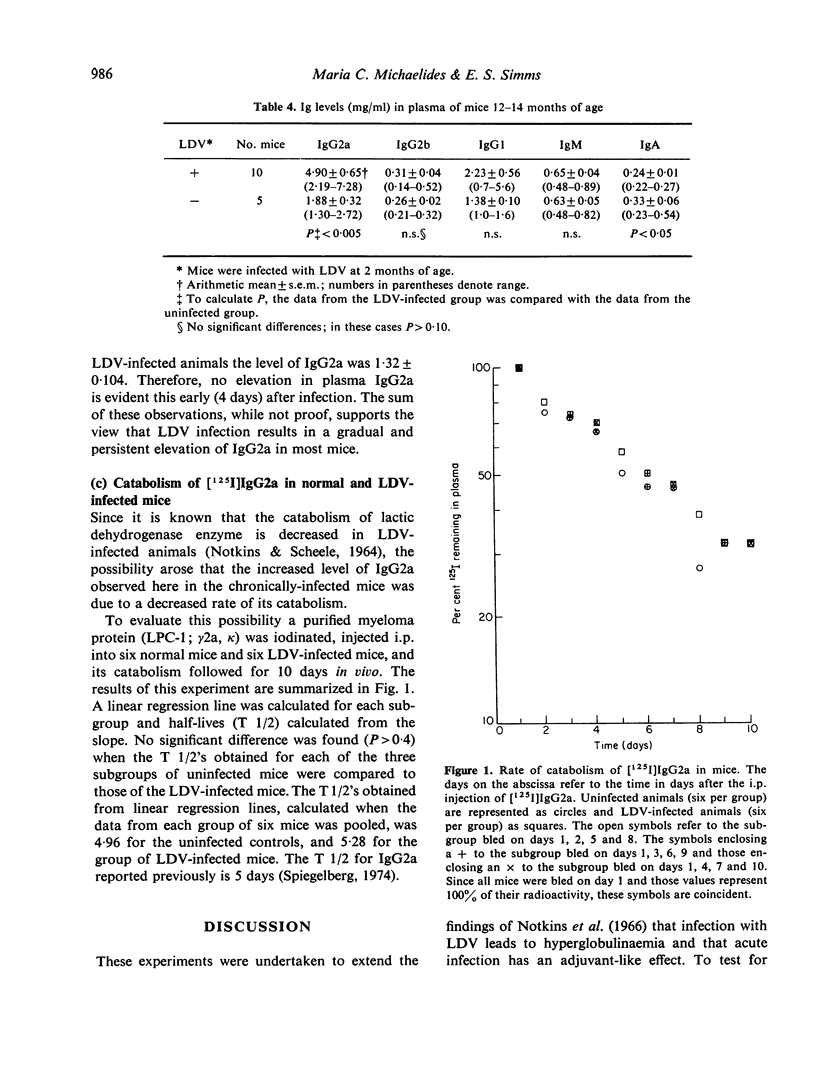
The humoral immune response to DNP-BGG of BALB/c mice acutely infected with lactic dehydrogenase virus (LDV) has been investigated. Virus-infected mice injected with antigen in saline exhibit a greater anti-DNP response than uninfected controls. When this antigen is presented in Freund's complete adjuvant (FCA) the anti-DNP response is greater than obtained with antigen in saline, but significant differences between infected and uninfected controls are not observed. These data are consistent with the view that acute LDV infection can have an adjuvant-like effect when this T-dependent antigen is introduced in saline. In addition, the effect of viral infection on plasma Ig class and subclass levels has been investigated. LDV infection leads to a gradual increase in plasma Ig concentration. This effect is restricted to the IgG2a subclass in most animals, but occasionally is restricted to IgG1. The mechanisms responsible for these changes have not been delineated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., ROBINSON A. G. FACTORS CONTROLLING SERUM GAMMA-GLOBULIN CONCENTRATION. J Exp Med. 1963 Nov 1;118:845–868. doi: 10.1084/jem.118.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Notkins A. L., Mergenhagen S. E. Inhibition of cellular immune reactions in mice infected with lactic dehydrogenase virus. Nature. 1969 Mar 1;221(5183):873–874. doi: 10.1038/221873a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Urban J. F., Jr, Ishizaka K. IgE formation in the rat following infection with Nippostrongylus brasiliensis. I. Proliferation and differentiation of IgE-bearing cells. Cell Immunol. 1976 Mar 15;22(2):248–261. doi: 10.1016/0008-8749(76)90027-7. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Michaelides M. C., Schlesinger S. Effect of acute or chronic infection with lactic dehydrogenase virus (LDV) on the susceptibility of mice to plasmacytoma MOPC-315. J Immunol. 1974 Apr;112(4):1560–1564. [PubMed] [Google Scholar]
- Michaelides M. C., Schlesinger S. Structural proteins of lactic dehydrogenase virus. Virology. 1973 Sep;55(1):211–217. doi: 10.1016/s0042-6822(73)81023-2. [DOI] [PubMed] [Google Scholar]
- NOTKINS A. L., SCHEELE C. IMPAIRED CLEARANCE OF ENZYMES IN MICE INFECTED WITH THE LACTIC DEHYDROGENASE AGENT. J Natl Cancer Inst. 1964 Oct;33:741–749. [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Rizzo A. A., Scheele C., Waldmann T. A. Elevated gamma-globulin and increased antibody production in mice infected with lactic dehydrogenase virus. J Exp Med. 1966 Feb 1;123(2):347–364. doi: 10.1084/jem.123.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Simms E. S., Eisen H. N. Subunit structure and number of combining sites of the immunoglobulin A myeloma protein produced by mouse plasmacytoma MOPC-315. Biochemistry. 1971 Nov 23;10(24):4359–4368. doi: 10.1021/bi00800a002. [DOI] [PubMed] [Google Scholar]



