Abstract
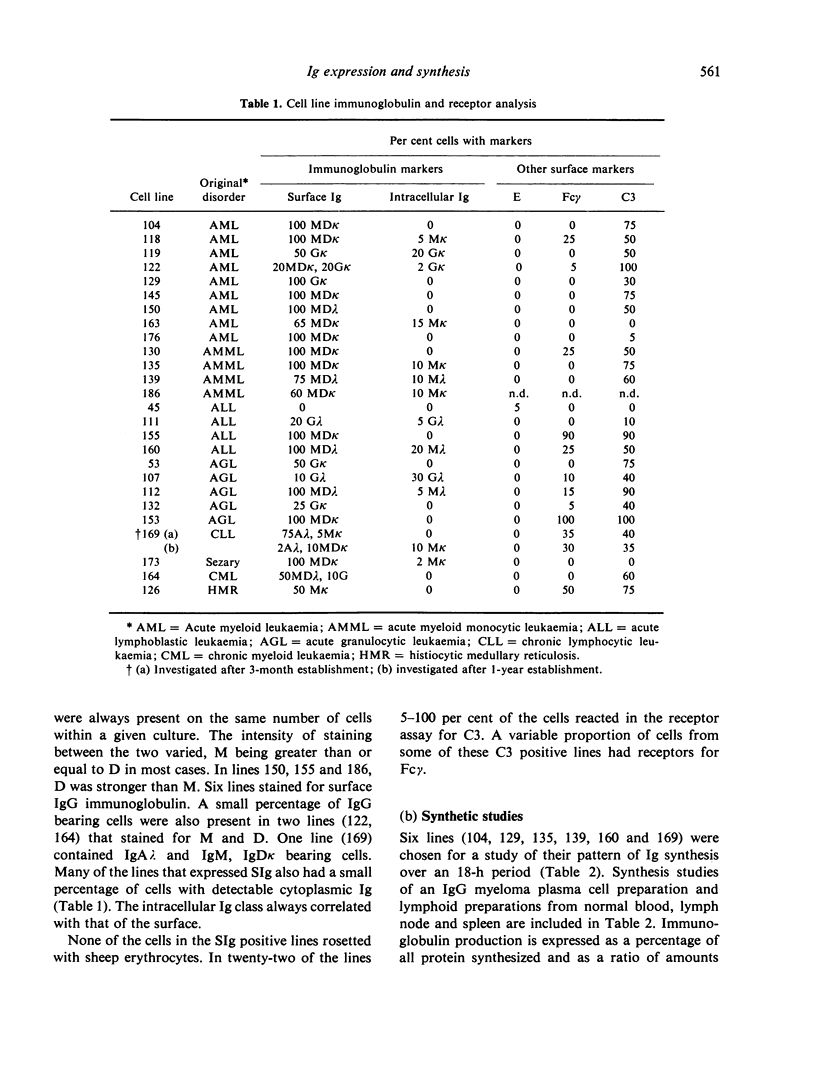
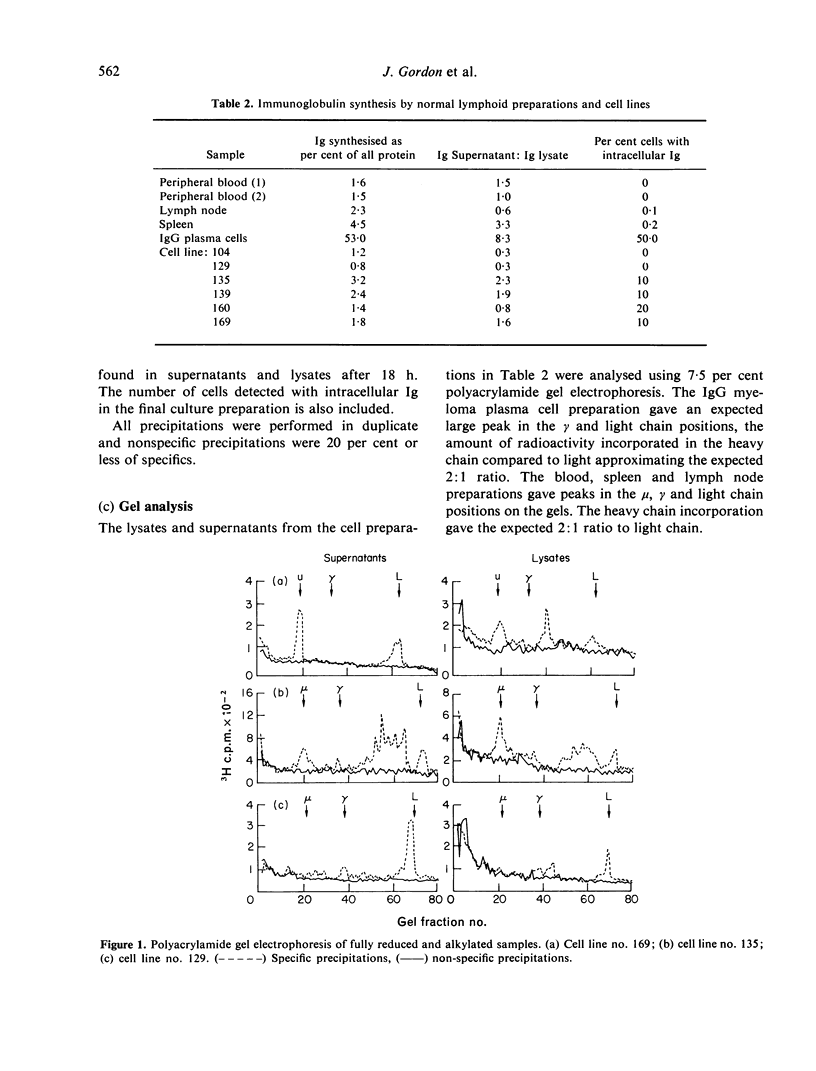
Twenty-six human cell lines derived from a variety of lymphoid and non-lymphoid malignancies, were investigated for their immunological markers, with special reference to the class of immunoglobulin expressed. Twenty-five of the lines stained positively for surface immunoglobulin and IgD together with IgM proved to be the major immunoglobulin classes on these cells. Six of the lines were chosen for a study of their immunoglobulin synthesis patterns over an 18-h period and the immunoglobulin produced was analysed on SDS-polyacrylamide gel electrophoresis. Patterns obtained from the cell lines were similar to that from normal lymph node lymphocytes and differed markedly to plasma cells. Two of the cell lines had abnormal immunoglobulin synthesis patterns characterized as free light chains in one case. The cell lines are evaluated for their usefulness as models of immunoglobulin synthesis and analogues of normal and neoplastic states.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Buxbaum J., Citronbaum R., Douglas S., Forni L., Melchers F., Pernis B., Stott D. IgM-producing tumors in the BALB-c mouse: a model for B-cell maturation. J Exp Med. 1974 Sep 1;140(3):742–763. doi: 10.1084/jem.140.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belpomme D., Minowada J., Moore G. E. Are some human lymphoblastoid cell lines established from leukemic tissues actually derived from normal leukocytes? Cancer. 1972 Jul;30(1):282–287. doi: 10.1002/1097-0142(197207)30:1<282::aid-cncr2820300139>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Preud'homme J. L., Scharff M. D. Variants of mouse myeloma cells that produce short immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3478–3482. doi: 10.1073/pnas.71.9.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley J. C., Smith J., Goldstone A. H., Emmines J., Hamblin J., Hough L. IgA and IgM cytoplastic inclusions in a series of cases of chronic lymphocytic leukaemia. Clin Exp Immunol. 1976 Jan;23(1):78–82. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., van Boxel J. A., Asofsky R., Paul W. E. Cell membrane IgD: demonstration of IgD on human lymphocytes by enzyme-catalyzed iodination and comparison with cell surface Ig of mouse, guinea pig, and rabbit. J Immunol. 1976 Apr;116(4):1173–1181. [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Kunkel H. G. Occurrence of surface IgM, IgD, and free light chains of human lymphocytes. J Exp Med. 1974 Feb 1;139(2):451–456. doi: 10.1084/jem.139.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Grey H. M., Pirofsky B. IgD: a major immunoglobulin on the surface of lymphocytes from patients with chronic lymphatic leukemia. J Immunol. 1974 May;112(5):1952–1954. [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Nilsson K., Pontén J. Classification and biological nature of established human hematopoietic cell lines. Int J Cancer. 1975 Feb 15;15(2):321–341. doi: 10.1002/ijc.2910150217. [DOI] [PubMed] [Google Scholar]
- Payne S. V., Jones D. B., Haegert D. G., Smith J. L., Wright D. H. T and B lymphocytes and Reed-Sternberg cells in Hodgkin's disease lymph nodes and spleens. Clin Exp Immunol. 1976 May;24(2):280–286. [PMC free article] [PubMed] [Google Scholar]
- Preud'homme J. L., Brouet J. C., Clauvel J. P., Seligmann M. Surface IgD in immunoproliferative disorders. Scand J Immunol. 1974;3(6):853–858. doi: 10.1111/j.1365-3083.1974.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Immunoglobulins on the surface of lymphoid cells in Waldenström's macroglobulinemia. J Clin Invest. 1972 Mar;51(3):701–705. doi: 10.1172/JCI106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Haegert D. B- and T-lymphocyte markers on transformed lymphocytes from mitogen-stimulated cultures of normal and CLL lymphocytes and on tonsil blasts. Clin Exp Immunol. 1974 Aug;17(4):547–560. [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Grundke-Iqbal I., Holmes K. V., Uhr J. W. Cell surface immunoglobulin. VII. Synthesis, shedding, and secretion of immunoglobulin by lymphoid cells of germ-free mice. J Exp Med. 1974 Apr 1;139(4):862–876. doi: 10.1084/jem.139.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]


