Abstract
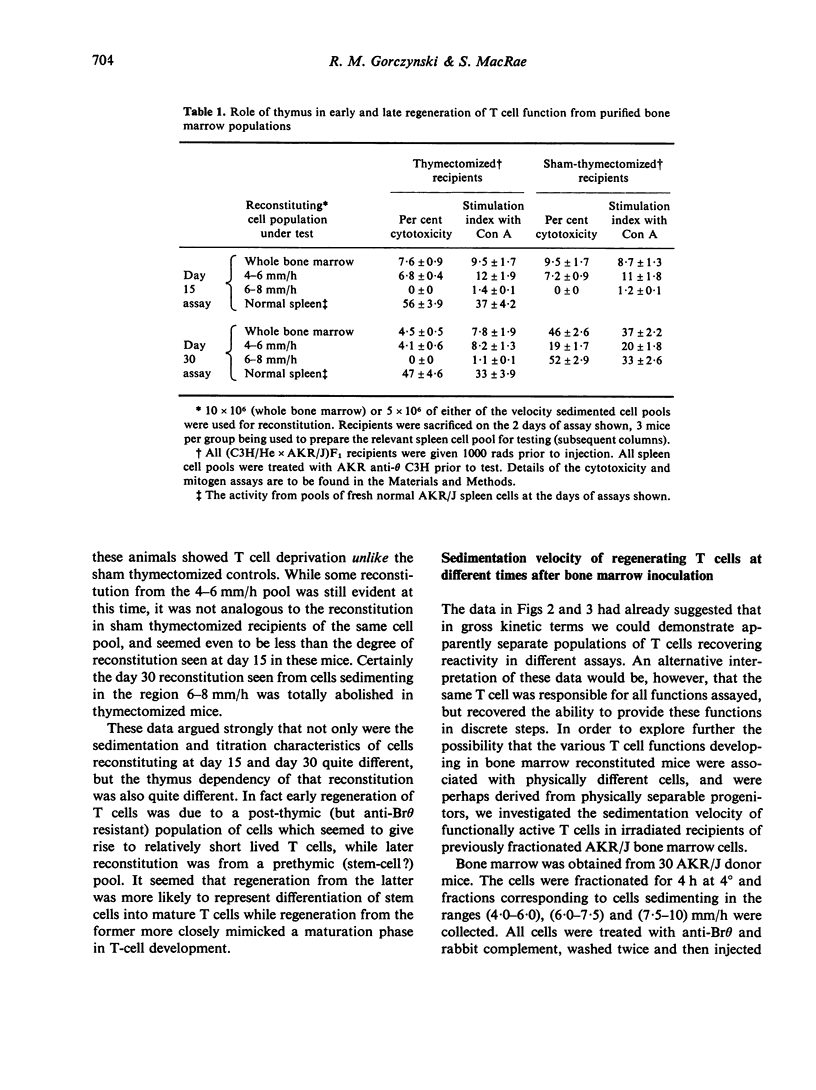
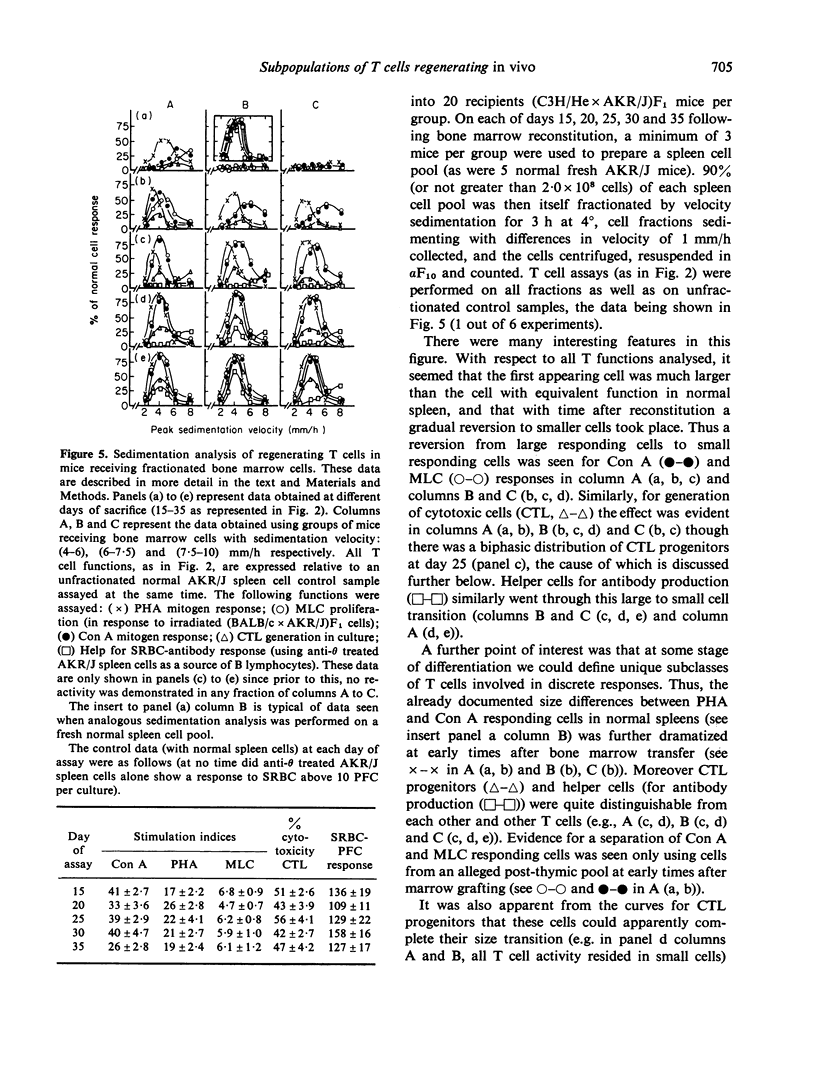
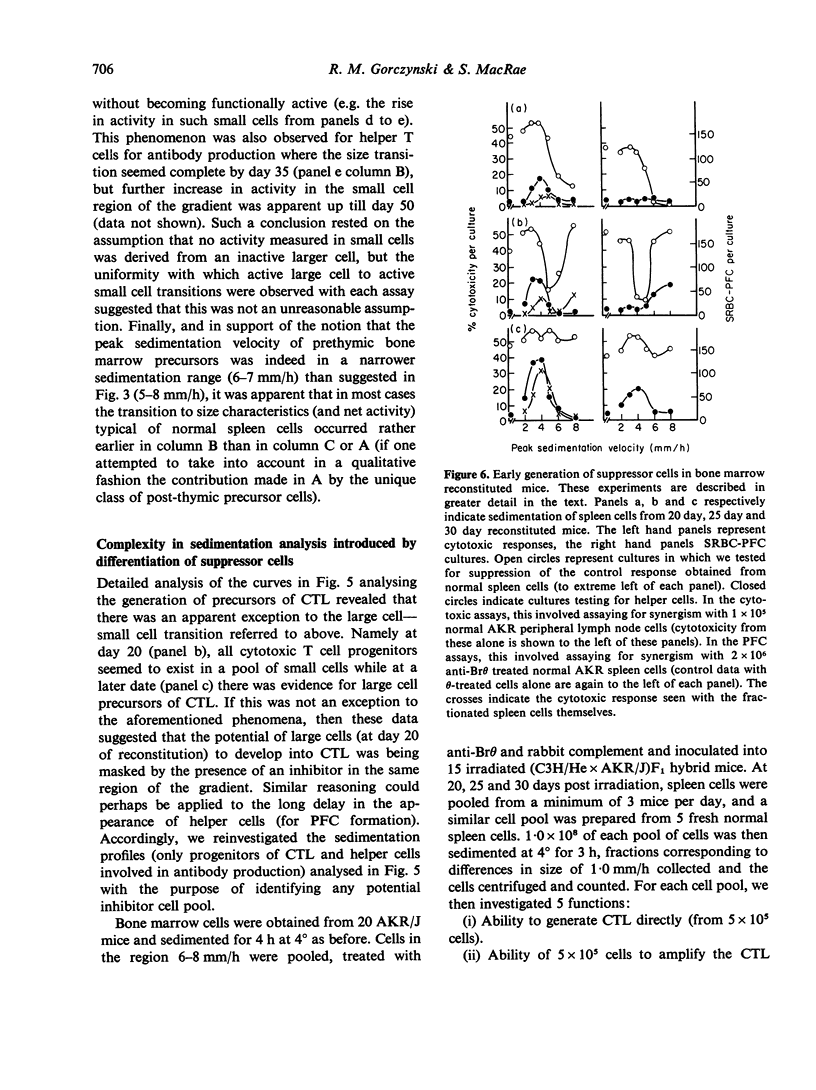
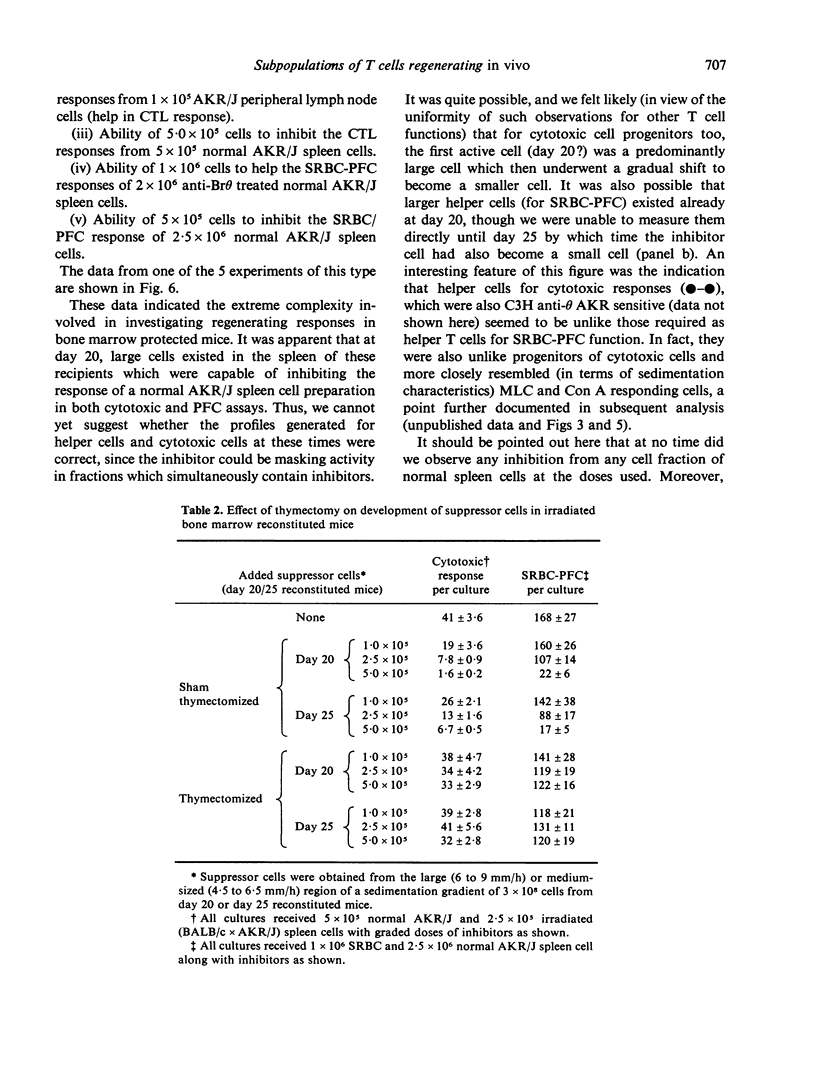
An investigation has been made of the development of various T cell functions in lethally irradiated mice reconstituted with anti-0 treated spleen or bone marrow cells. Evidence is presented to show that both organs contain a post-thymic precursor pool able to regenerate by 15 days limited T cell responses in thymectomized recipients. A prethymic pool also exists in each organ able to regenerate, at a later date, first a suppressor T cell population and probably later, mature functional T cells involved in helper functions and cell mediated lympholysis. The spleen is apparently a better source of precursors of the suppressor cells than bone marrow, while a poorer source of precursors of the other T cell functions. All T cell functions investigated apparently first appear in large cells which undergo a reversion to small cells without necessarily maturing to their full potential reactivity. By following the kinetics of appearance of T cell functions, and the physical parameters of the cells with which these functions are associated, it is shown that PHA responding and Con A responding cells, cytotoxic T cell progenitors, helper T cells for antibody production and helper T cells for cytotoxicity induction can all at some stage of differentiation be separated from one another.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALNER H., DE VRIES M. J., VAN BEKKUMD SECONDARY DISEASE IN RAT RADIATION CHIMERAS. J Natl Cancer Inst. 1964 Feb;32:419–459. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Simpson E., Sato V. L., Fathman C. G., Herzenberg L. A. Characterization of subpopulations of T lymphocytes. I. Separation and functional studies of peripheral T-cells binding different amounts of fluorescent anti-Thy 1.2 (theta) antibody using a fluorescence-activated cell sorter (FACS). Cell Immunol. 1975 Jan;15(1):180–196. doi: 10.1016/0008-8749(75)90174-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Patterson C. K. Induction of theta-positive lymphocytes and lymphoblasts in mouse bone marrow by mitogens. J Immunol. 1975 Jan;114(1 Pt 2):374–376. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Beverley P. C., Dunkley M., Kontiainen S. Different Ly antigen phenotypes of in vitro induced helper and suppressor cells. Nature. 1975 Dec 18;258(5536):614–616. doi: 10.1038/258614a0. [DOI] [PubMed] [Google Scholar]
- Frelinger J. A., Niederhuber J. E., Shreffler D. C. Effects of anti-Ia sera on mitogenic responses. III. Mapping the genes controlling the expression of Ia determinants on concanavalin A-reactive cells to the I-J subregion of the H-2 gene complex. J Exp Med. 1976 Oct 1;144(4):1141–1146. doi: 10.1084/jem.144.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Evidence for in vivo protection against murine-sarcoma virus-induced tumors by T lymphocytes from immune animals. J Immunol. 1974 Feb;112(2):533–539. [PubMed] [Google Scholar]
- Gorczynski R. M. Heteroantisera prepared against B lymphocytes at different stages of differentiation. I. Preparation of sera and cytotoxicity to lymphoid cells from different organs. Immunology. 1977 May;32(5):709–715. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M. Heteroantisera prepared against B lymphocytes at different stages of differentiation. II. Functional analysis of cytotoxicity to different B-cell populations. Immunology. 1977 May;32(5):717–729. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Rittenberg M. B. Stimulation of early protein synthesis as an assay of immune reactivity: analysis of the cells responding to mitogens and alloantigens. J Immunol. 1974 Jan;112(1):47–55. [PubMed] [Google Scholar]
- Huber B., Cantor H., Shen F. W., Boyse E. A. Independent differentiative pathways of Ly1 and Ly23 subclasses of T cells. Experimental production of mice deprived of selected T-cell subclasses. J Exp Med. 1976 Oct 1;144(4):1128–1133. doi: 10.1084/jem.144.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Hamashima Y., Masuda T. Immunological restoration of both thymectomised and athymic nude mice by a thymus factor. Nature. 1975 Nov 27;258(5533):335–337. doi: 10.1038/258335a0. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Gorczynski R. M., Lafleur L., MacDonald H. R., Phillips R. A. Cell separation analysis of B and T lymphocyte differentiation. Transplant Rev. 1975;25:59–97. doi: 10.1111/j.1600-065x.1975.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M., Israel E., Gery I. Antigenic properties of subsets of splenic T lymphocytes responding to lectins. Immunology. 1976 Jun;30(6):865–872. [PMC free article] [PubMed] [Google Scholar]
- Sharon R., McMaster P. R., Kask A. M., Owens J. D., Paul W. E. DNP-Lys-ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975 May;114(5):1585–1589. [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Bean M. A., Old L. J., Oettgen H. F. Ly phenotype of cytotoxic T cells for syngeneic tumor. J Exp Med. 1976 Oct 1;144(4):1116–1120. doi: 10.1084/jem.144.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K. Separation methods for lymphocyte populations. Contemp Top Mol Immunol. 1974;3:161–203. doi: 10.1007/978-1-4684-2838-4_7. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes: II. Mitogen responsiveness of T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Nov 1;142(5):1041–1051. doi: 10.1084/jem.142.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]


