Abstract
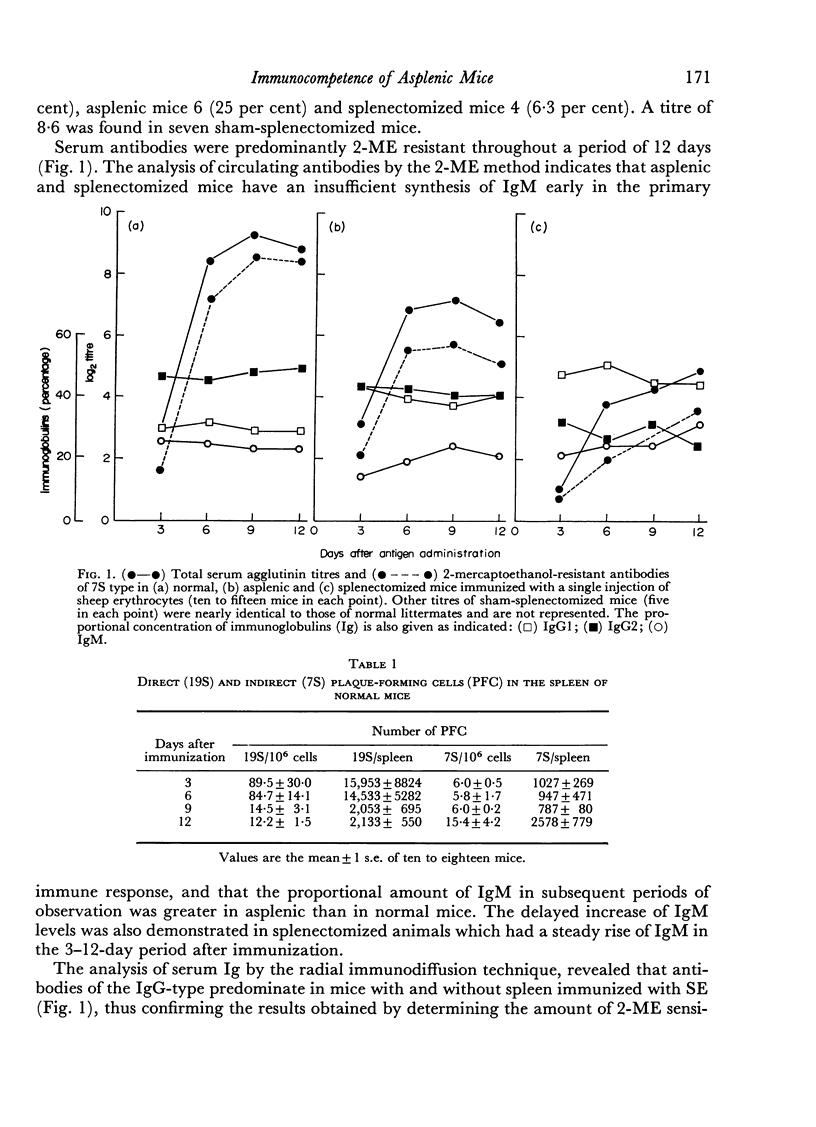
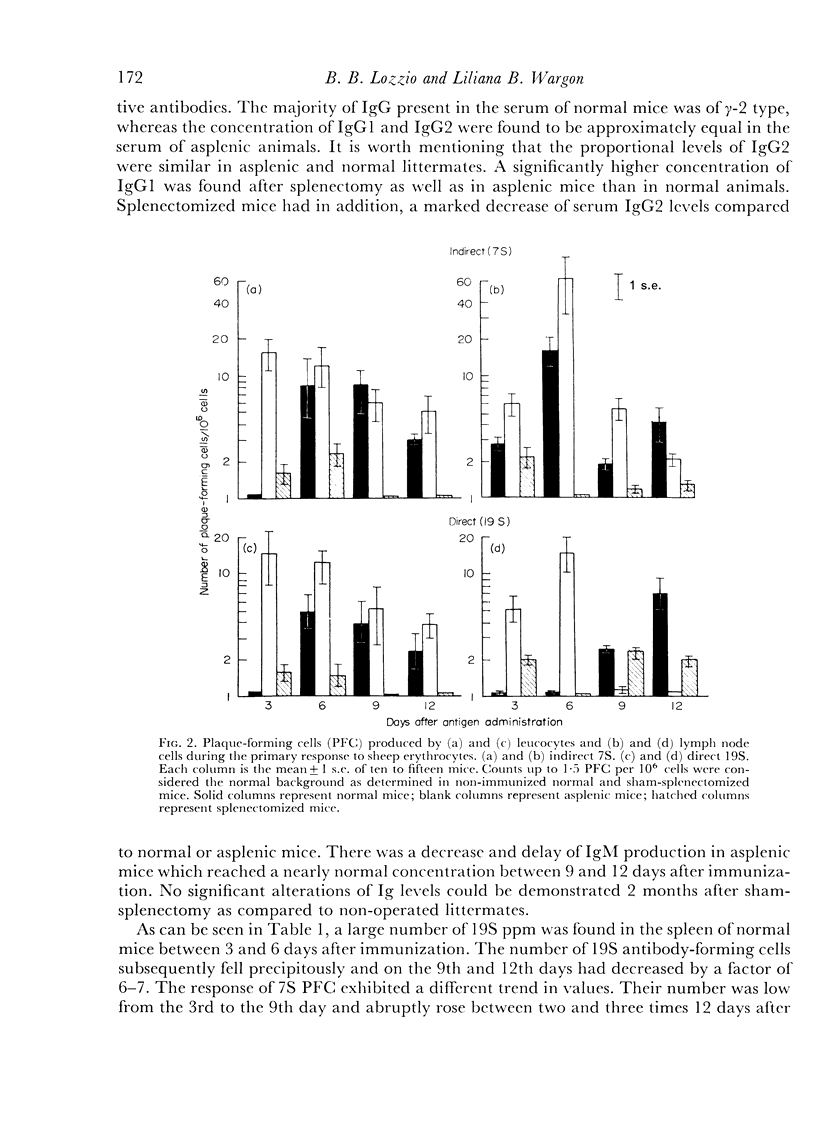
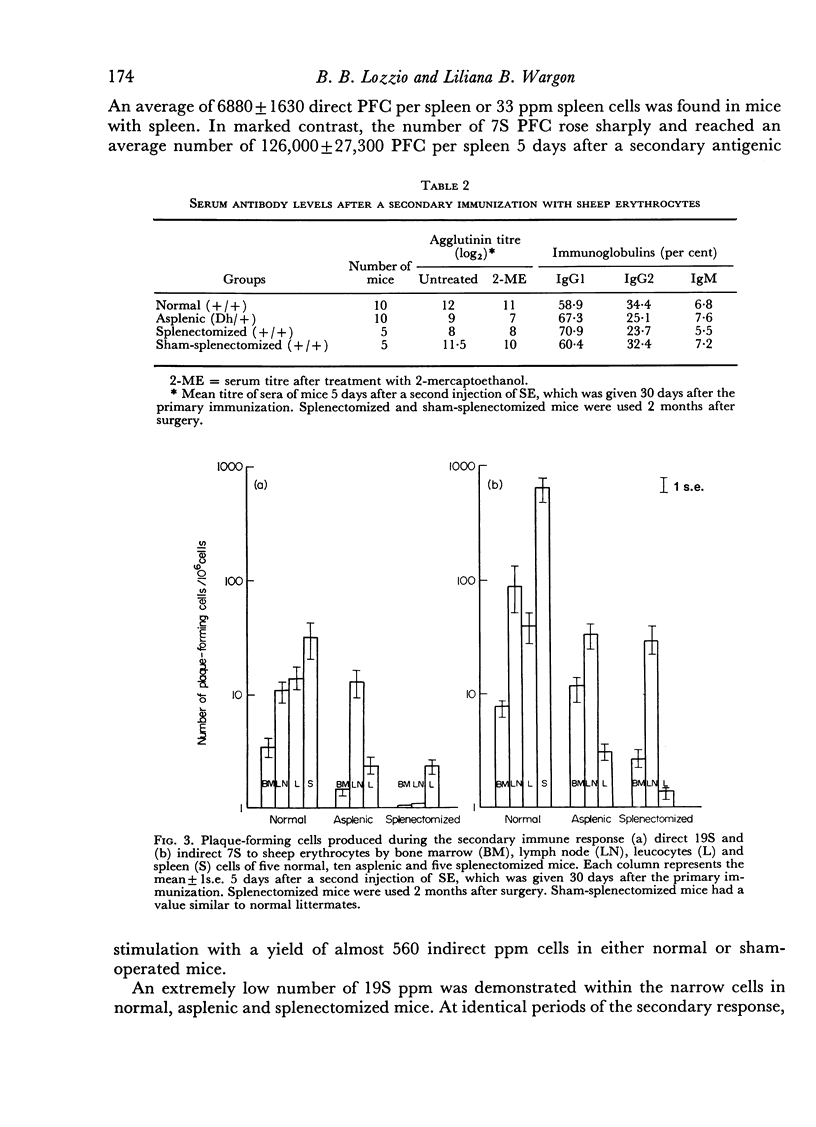
Antibody formation and cellular immunity were studied in congenitally asplenic mice challenged with sheep erythrocytes (SE) and transplants of skin and spleen. Asplenic mice had a significantly lower antibody production than normal animals, albeit higher than splenectomized littermates in the primary and secondary immune responses. The serum levels of immunoglobulin (Ig) M of asplenic mice were deminished after a primary immunization and normal in the secondary response. Although the serum concentration of IgG2 was normal after a single antigenic stimulation, there was a reduction of IgG2 serum levels during the secondary response. The serum concentration of IgG1 was significantly higher in asplenic than normal mice. The number of 19S PFC was markedly reduced throughout the lymphopoietic system of asplenic mice which had a large number of 19S and 7S PFC in the blood stream, when compared to normal littermates, after a single antigen injection. During the secondary immune response there was a great improvement of the number of PFC in lymph nodes of normal and splenectomized mice but not in that of asplenic mice. The number of 7S PFC in other lymphoid tissues of asplenic mice was markedly diminished. Hereditarily asplenic mice had normal cellular immunity as indicated by normal rejection times of spleen and skin allografts. The results are consistent with the concept of decreased antibody production associated with asplenia and demonstrated the important function of the spleen during embryogenesis to achieve normal humoral immunity in adult life.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battisto J. R., Borek F., Bucsi R. A. Splenic determination of immunocompetence: influence on other lymphoid organs. Cell Immunol. 1971 Dec;2(6):627–633. doi: 10.1016/0008-8749(71)90010-4. [DOI] [PubMed] [Google Scholar]
- Battisto J. R., Cantor L. C., Borek F., Goldstein A. L., Cabrerra E. Immunoglobulin synthesis in hereditarily spleenless mice. Nature. 1969 Jun 21;222(5199):1196–1198. doi: 10.1038/2221196a0. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Perkins E. H., Makinodan T. Further characterization of the lymphoid cell transfer system for the study of antigen-sensitive progenitor cells. J Immunol. 1968 Nov;101(5):963–972. [PubMed] [Google Scholar]
- Brown G. L., Carpenter P. L. Characteristics of cytophilic antibody in the mouse. Infect Immun. 1971 May;3(5):637–641. doi: 10.1128/iai.3.5.637-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucsi R. A., Borek F., Battisto J. R. Splenic replenishment of synergistic ability to bone marrow and thymic cells of neonatally splenectomized CBA mice. J Exp Med. 1972 Oct 1;136(4):761–768. doi: 10.1084/jem.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H. Hyposplenism: an inquiry into normal functions of the spleen. Annu Rev Med. 1963;14:349–370. doi: 10.1146/annurev.me.14.020163.002025. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., La Via M. F. Effect of splenectomy on primary and secondary response to sheep erythrocytes in rats. Proc Soc Exp Biol Med. 1967 Feb;124(2):571–573. doi: 10.3181/00379727-124-31794. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Smith J. B., Mercer E. H. Antibody formation by single cells from lymph nodes and efferent lymph of sheep. J Exp Med. 1966 Oct 1;124(4):701–714. doi: 10.1084/jem.124.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMESHEK W. Hypersplenism. Bull N Y Acad Med. 1955 Feb;31(2):113–136. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- HULLIGER L., SORKIN E. Synthesis of antibodies by blood leucocytes of the rabbit. Nature. 1963 Apr 20;198:299–299. doi: 10.1038/198299a0. [DOI] [PubMed] [Google Scholar]
- Halasa J. Plaque forming cells in the spleen, lymph and blood. Folia Microbiol (Praha) 1968;13(4):253–258. doi: 10.1007/BF02909610. [DOI] [PubMed] [Google Scholar]
- Hauptfeldová-DolejskovI, Nouza K., Matousek V., Hauptfeld M. Neonatal splenectomy in micel. Folia Biol (Praha) 1969;15(4):288–299. [PubMed] [Google Scholar]
- Hulliger L., Sorkin E. Formation of specific antibody by circulating cells. Immunology. 1965 Oct;9(4):391–401. [PMC free article] [PubMed] [Google Scholar]
- KEARNEY R., HALLIDAY W. J. ENUMERATION OF ANTIBODY-FORMING CELLS IN THE PERIPHERAL BLOOD OF IMMUNIZED RABBITS. J Immunol. 1965 Jul;95:109–112. [PubMed] [Google Scholar]
- LANDY M., SANDERSON R. P., BERNSTEIN M. T., JACKSON A. L. ANTIBODY PRODUCTION BY LEUCOCYTES IN PERIPHERAL BLOOD. Nature. 1964 Dec 26;204:1320–1321. doi: 10.1038/2041320a0. [DOI] [PubMed] [Google Scholar]
- Lozzio B. B., Comas F. V. Biological effects of pokeweed mitogen. Int Arch Allergy Appl Immunol. 1969;36(3):266–281. doi: 10.1159/000230748. [DOI] [PubMed] [Google Scholar]
- Lozzio B. B. Hematopoiesis in congenitally asplenic mice. Am J Physiol. 1972 Feb;222(2):290–295. doi: 10.1152/ajplegacy.1972.222.2.290. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- NATIONAL ACADEMY OF SCIENCES: Abstracts of Papers Presented at the Annual Meeting, April 23-25, 1951 Washington, D. C. Science. 1951 Apr 27;113(2939):473–484. doi: 10.1126/science.113.2939.473. [DOI] [PubMed] [Google Scholar]
- Nakov L., Sejkorová J., Nouza K. Neonatal splenectomy in rats. Folia Biol (Praha) 1969;15(6):439–452. [PubMed] [Google Scholar]
- PERKINS E. H., ROBINSON M. A., MAKINODAN T. Agglutinin response, a function of cell number. J Immunol. 1961 May;86:533–537. [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- Rabin B. S., Rose N. R. Selective release of antibody forming cells into the blood. Cell Immunol. 1973 Jun;7(3):389–395. doi: 10.1016/0008-8749(73)90203-7. [DOI] [PubMed] [Google Scholar]
- Roseman J. M., Leserman L. D., Fitch F. W., Rowley D. A. Do antibody-forming cells circulate in the blood? J Immunol. 1969 Apr;102(4):1002–1007. [PubMed] [Google Scholar]
- Sorkin E., Landy M. Antibody production by blood leucocytes. Experientia. 1965 Nov 15;21(11):677–680. doi: 10.1007/BF02144077. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H. Functions of the spleen in immunity; presidential address. Am J Trop Med Hyg. 1956 May;5(3):391–410. doi: 10.4269/ajtmh.1956.5.391. [DOI] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G. The dynamics of hemolysin formation in intact and splenectomized rabbits. J Infect Dis. 1950 Jul-Aug;87(1):37–62. doi: 10.1093/infdis/87.1.37. [DOI] [PubMed] [Google Scholar]


