Abstract
The functional capacity of lymphoid cells has been determined in amyloid-susceptible CBA/J and amyloid-resistant A/J mice. Following multiple casein injections mitogen responses to phytohaemagglutinin (PHA), concanavalin A (Con-A), pokeweed mitogen (PKW) and lipopolysaccharide (LPS) were significantly depressed in spleen cell suspensions of the A/J strain. Mitogen responses to PHA, Con-A and PKW were also reduced in casein-treated CBA/J animals, but improved during the active phase of amyloid deposition. The response to LPS was not affected in pre-amyloid and amyloid CBA/J animals. Spleen cells from caseintreated CBA/J mice responded normally to allogeneic cells from BALB/c and A/J mice; on the other hand graft-versus-host reactions in BALB/c mice receiving spleen cells from pre-amyloid and amyloid CBA/J mice were markedly impaired. The marked depression of certain T-cell populations, plus the maintenance of normal B-cell function in the CBA/J mice, suggest that disturbances of immunoregulatory mechanisms may be a critical step in the pathogenesis of experimental amyloid disease.
Full text
PDF



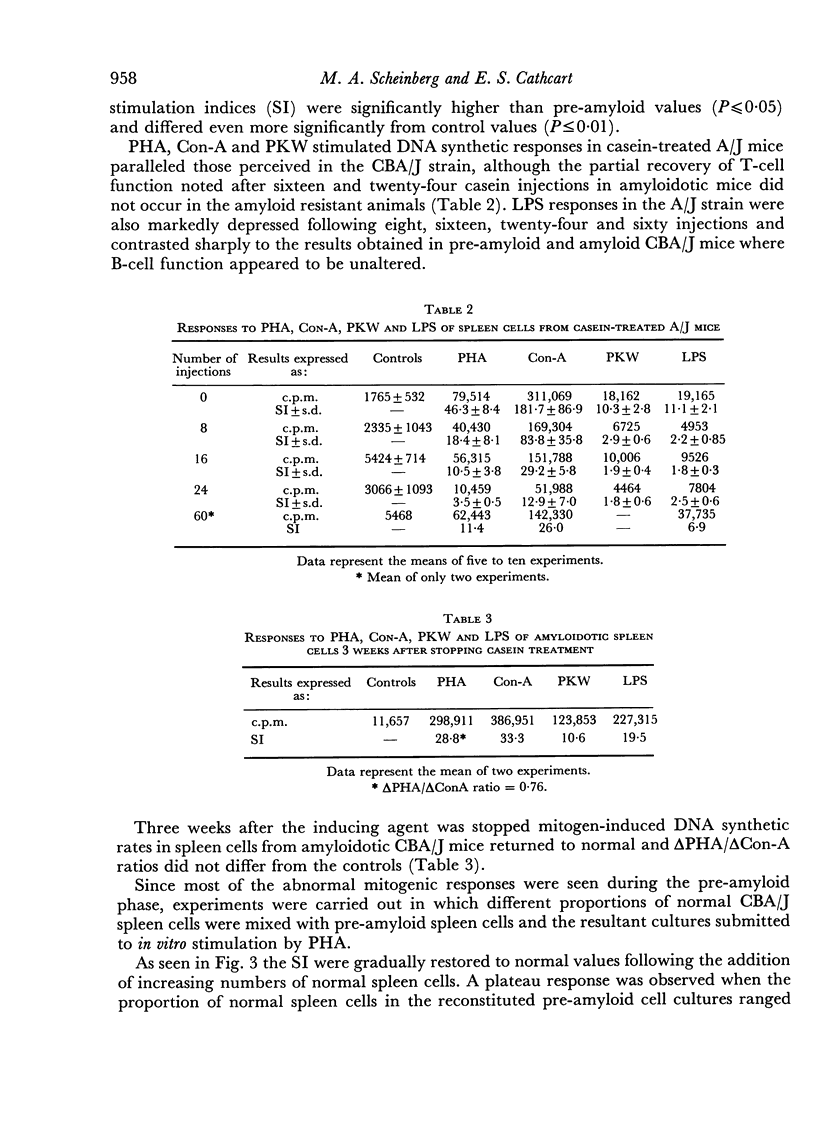
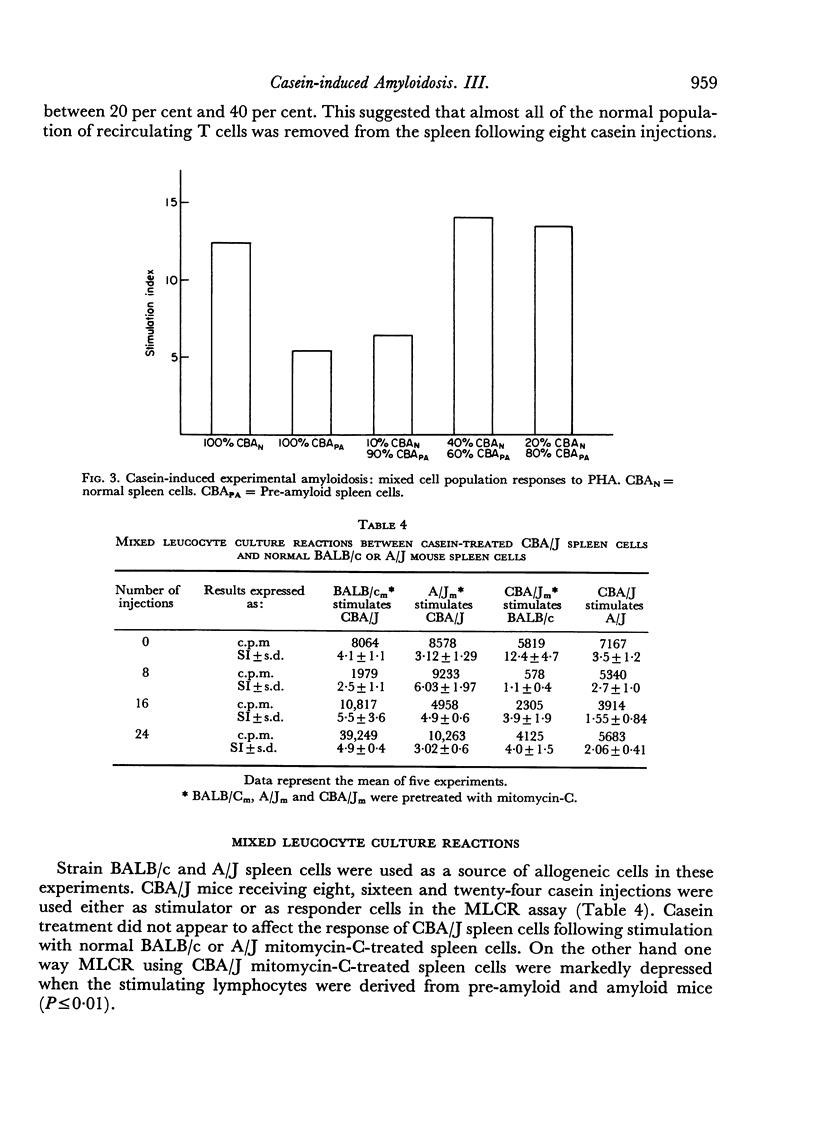




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Voynow N. K. One-way stimulation in mixed leukocyte cultures. Science. 1966 Jul 29;153(3735):545–547. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- Bari W. A., Pettengill O. S., Sorenson G. D. Electron microscopy and electron microscopic autoradiography of splenic cell cultures from mice with amyloidosis. Lab Invest. 1969 Mar;20(3):234–242. [PubMed] [Google Scholar]
- Barth W. F., Gordon J. K., Willerson J. T. Amyloidosis induced in mice by Escherichia coli endotoxin. Science. 1968 Nov 8;162(3854):694–695. doi: 10.1126/science.162.3854.694. [DOI] [PubMed] [Google Scholar]
- Bennett M. Graft-versus-host reactions in mice. I. Kinetic and immunogenetic studies of alloantigen-sensitive units of lymphoid tissue. Transplantation. 1971 Feb;11(2):158–169. [PubMed] [Google Scholar]
- Cathcart E. S., Mullarkey M., Cohen A. S. Cellular immunity in casein-induced amyloidosis. Immunology. 1971 Jun;20(6):1001–1008. [PMC free article] [PubMed] [Google Scholar]
- Claesson M. H., Hardt F. Quantitative studies on the decay of lymphoid cells during the development of casein-induced murine amyloidosis. Acta Pathol Microbiol Scand A. 1972;80(1):125–133. doi: 10.1111/j.1699-0463.1972.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Clerici E., Pierpaoli W., Romussi M. Experimental amyloidodis in immunity. Pathol Microbiol (Basel) 1965;28(5):806–815. doi: 10.1159/000161835. [DOI] [PubMed] [Google Scholar]
- Druet R. L., Janigan D. T. Experimental amyloidosis. Amyloid induction with a soluble protein antigen in intact, bursectomized and thymectomized chickens. Am J Pathol. 1966 Dec;49(6):1103–1123. [PMC free article] [PubMed] [Google Scholar]
- Druet R. L., Janigan D. T. Experimental amyloidosis. Rates of induction, lymphocyte depletion and thymic atrophy. Am J Pathol. 1966 Nov;49(5):911–929. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- KELLUM M. J., SUTHERLAND D. E., ECKERT E., PETERSON R. D., GOOD R. A. WASTING DISEASE, COOMBS-POSITIVITY, AND AMYLOIDOSIS IN RABBITS SUBJECTED TO CENTRAL LYMPHOID TISSUE EXTIRPATION AND IRRADIATION. Int Arch Allergy Appl Immunol. 1965;27:6–26. doi: 10.1159/000229594. [DOI] [PubMed] [Google Scholar]
- Muckle T. J. Impaired immunity in the etiology of amyloidosis: a speculative review. Isr J Med Sci. 1968 Sep-Oct;4(5):1020–1034. [PubMed] [Google Scholar]
- Ram J. S., DeLellis R. A., Glenner G. G. Amyloid. 3. A method for rapid induction of amyloidosis in mice. Int Arch Allergy Appl Immunol. 1968;34(2):201–204. [PubMed] [Google Scholar]
- Ranlov P., Hardt F. In vitro evaluation of cell-mediated immunity in mice: experiments with soluble and cellular antigens in a spleen-thymus cell leucocyte migration test (LMT). Clin Exp Immunol. 1971 Feb;8(2):163–171. [PMC free article] [PubMed] [Google Scholar]
- Ranlov P., Jensen E. Homograft reaction in amyloidotic mice. Acta Pathol Microbiol Scand. 1966;67(2):161–164. doi: 10.1111/apm.1966.67.2.161. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. An analysis of the close relationship of lysosomes to early deposits of amyloid. Ultrastructural evidence in experimental mouse amyloidosis. Am J Pathol. 1973 Oct;73(1):97–114. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Willerson J. T., Asofsky R., Barth W. F. Experimental murine amyloid. IV. Amyloidosis and immunoglobulins. J Immunol. 1969 Oct;103(4):741–749. [PubMed] [Google Scholar]



