Abstract
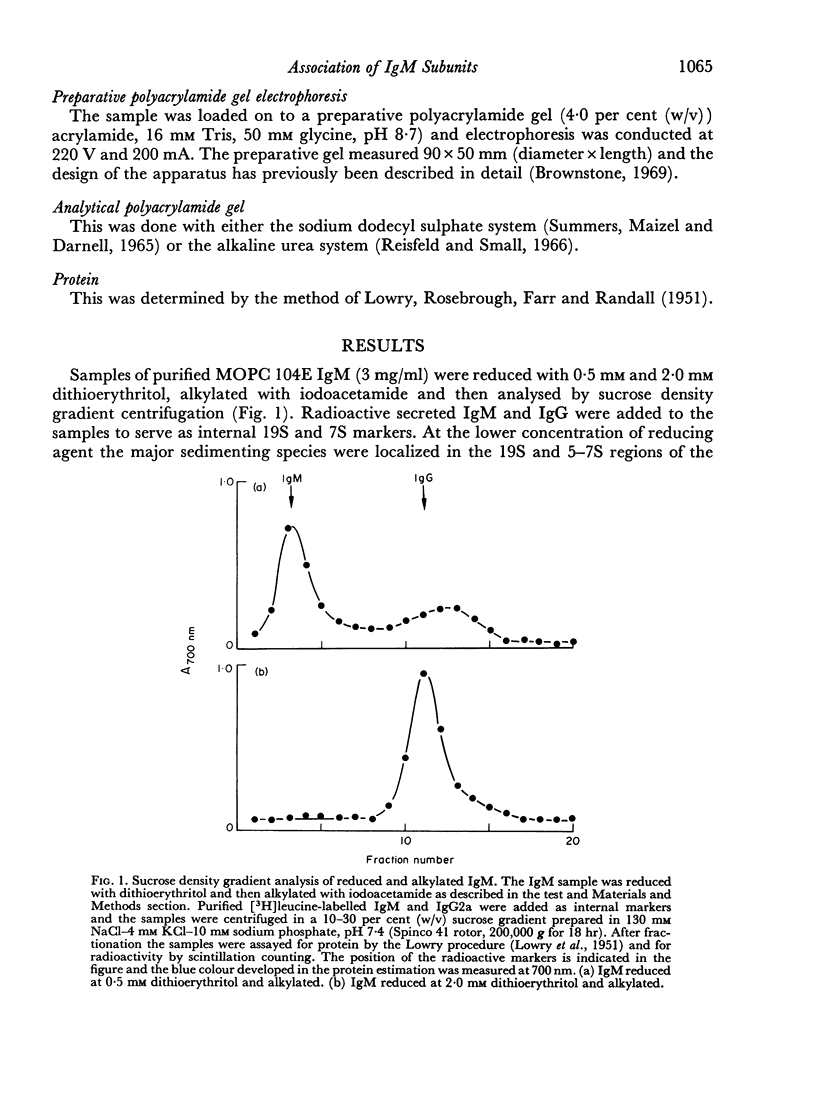
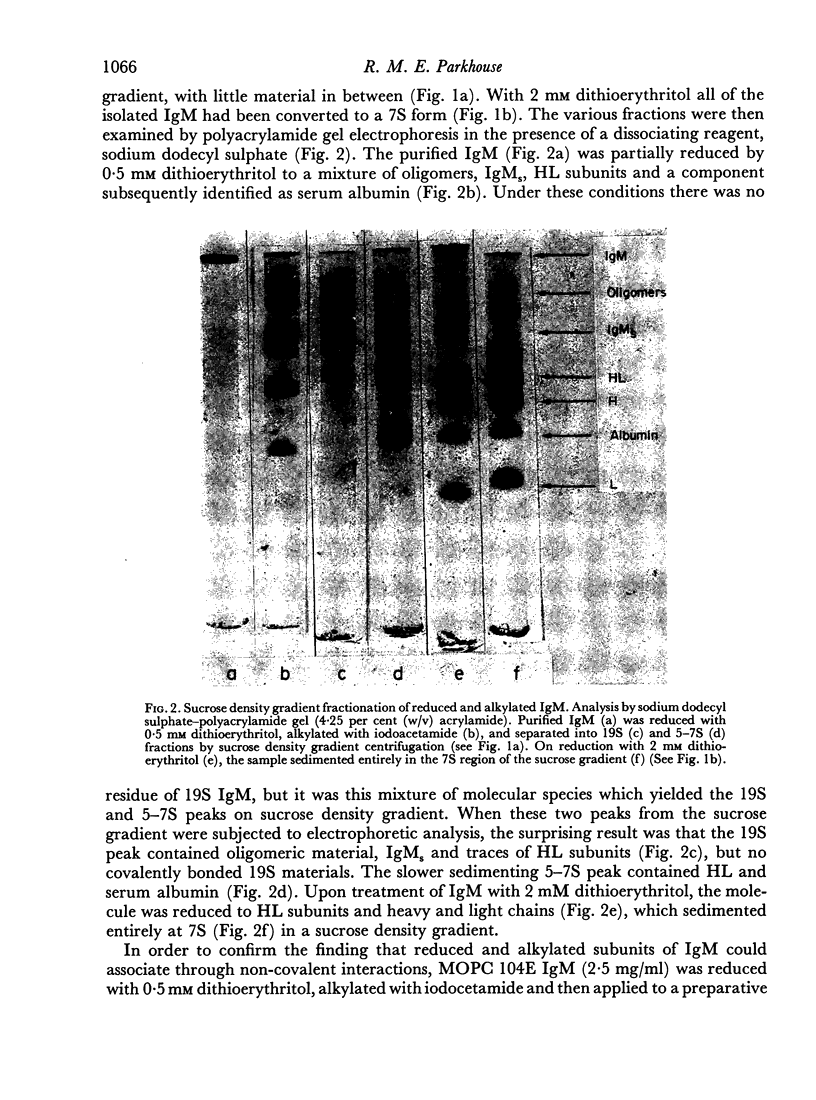
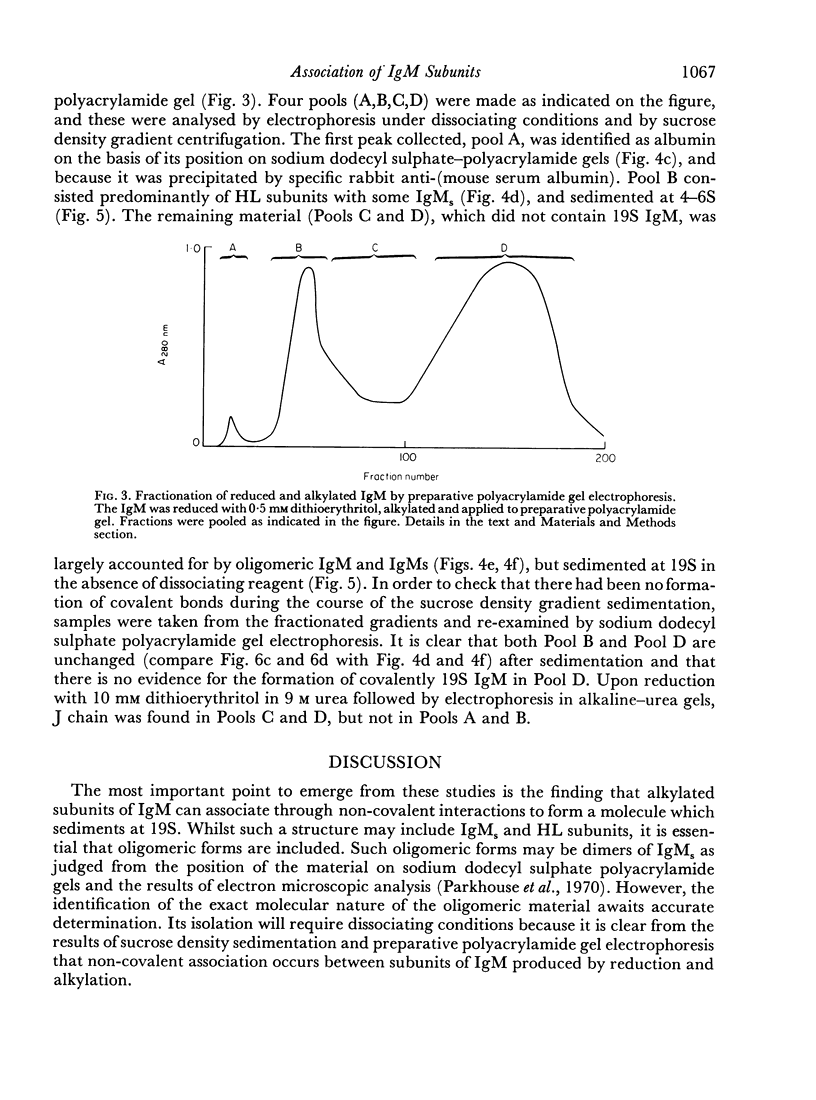
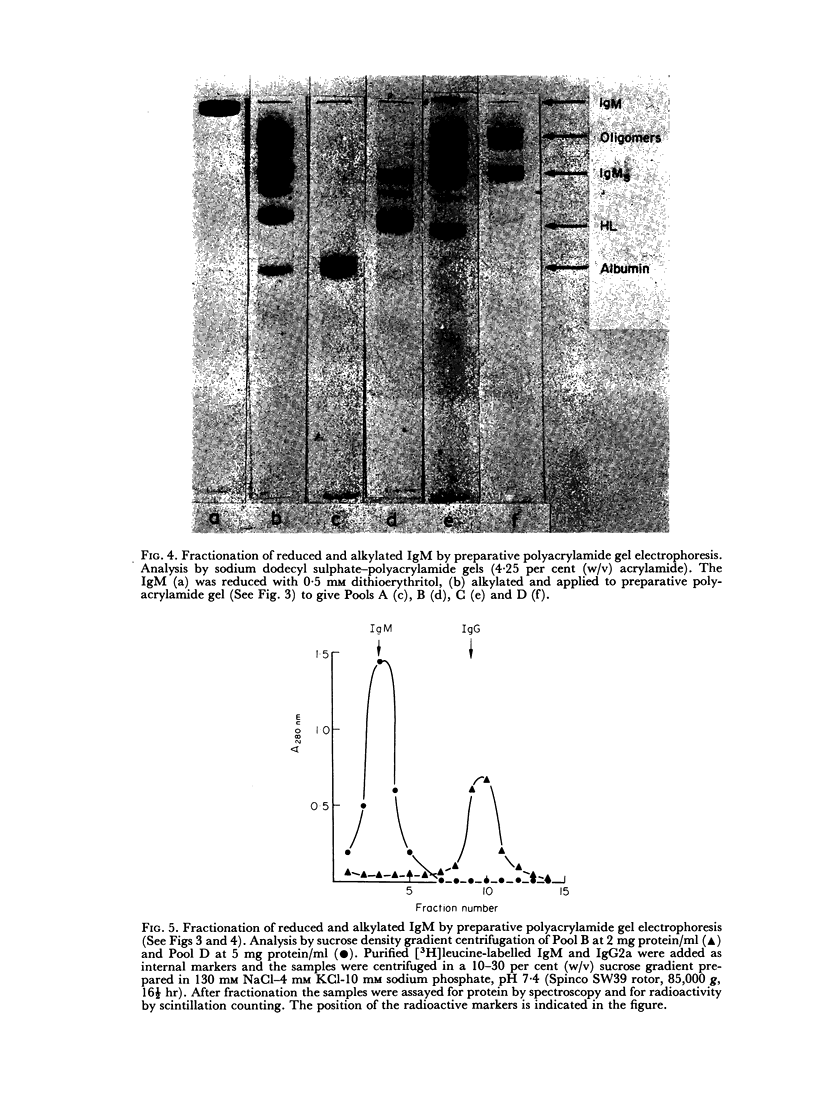
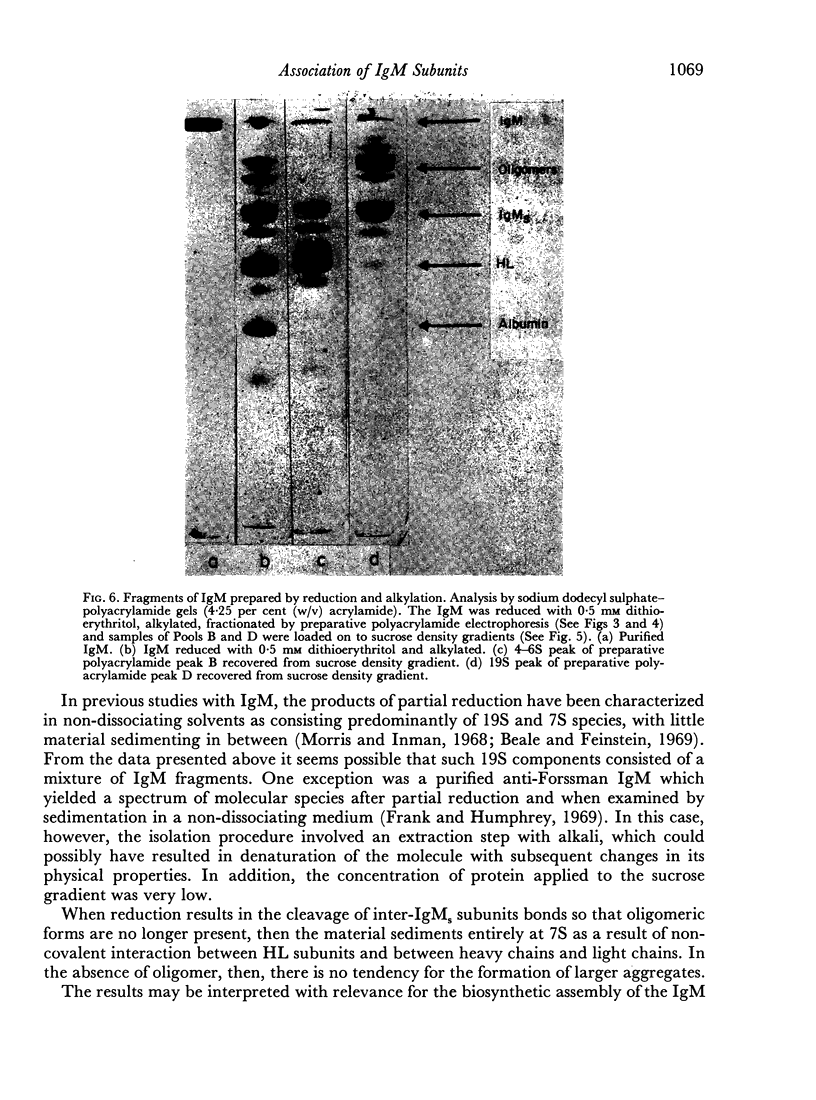
Purified IgM isolated from the serum of mice bearing the transplantable plasmacytoma MOPC 104E was reduced and alkylated and then analysed by sucrose density gradient centrifugation and by sodium dodecyl sulphate—polyacrylamide gel electrophoresis. On partial reduction a mixture of IgM subunits was obtained in the absence of covalently linked 19S IgM. When examined under dissociating conditions this mixture was found to consist of disulphide-linked 7S subunits (IgMs), small amounts of HL subunits and oligomeric IgM of a size intermediate between monomeric and pentameric IgM. In the absence of a dissociating agent and on sucrose density gradient, however, the mixture resolved into a 19S and a 7S peak. The 19S peak consisted primarily of oligomeric IgM and IgMs with small amounts of HL subunits. Thus alkylated IgMs and HL subunits of IgM can associate through non-covalent forces to form a molecule sedimenting at 19S, providing oligomeric forms are present. In the absence of oligomeric forms, IgMs, HL subunits and heavy and light chains sediment at about 7S. The products of partial reduction which sediment at 7S and 19S could also be isolated by preparative polyacrylamide gel electrophoresis. When this was done J chain was absent in the former and present in the latter, raising the possibility that J chain does not disulphide bond to each of the five IgMs subunits, constituting an IgM molecule. Thus, within cells secreting IgM, J chain would be expected to mediate the formation of an oligomeric form of IgM. Once the oligomeric structure has been assembled, then non-covalent forces between this and IgMs subunits will cause the formation of a 19S structure, thereby facilitating the final assembly through disulphide bonds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Parkhouse R. M. Assembly of immunoglobulin M. Blocked thiol groups of intracellular 7S subunits. Biochem J. 1971 Jul;123(4):629–634. doi: 10.1042/bj1230629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D., Feinstein A. Studies on the reduction of a human 19S immunoglobulin M. Biochem J. 1969 Apr;112(2):187–194. doi: 10.1042/bj1120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone A. D. A versatile system for preparative electrophoresis in acrylamide gel. Anal Biochem. 1969 Jan;27(1):25–46. doi: 10.1016/0003-2697(69)90216-4. [DOI] [PubMed] [Google Scholar]
- Chapuis R. M., Koshland M. E. Mechanism of IgM polymerization. Proc Natl Acad Sci U S A. 1974 Mar;71(3):657–661. doi: 10.1073/pnas.71.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavin S. I., Franklin E. C. Studies on antigen-binding activity of macroglobulin antibody subunits and their enzymatic fragments. J Biol Chem. 1969 Mar 10;244(5):1345–1352. [PubMed] [Google Scholar]
- Cooper A. G. Hemagglutinating 7S subunits of 19S cold agglutinins. Science. 1967 Aug 25;157(3791):933–935. doi: 10.1126/science.157.3791.933. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA) and immunoglobulin M (IgM). Requirement for J chain and a disulphide-exchanging enzyme for polymerization. Biochem J. 1973 Nov;136(3):597–606. doi: 10.1042/bj1360597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder F. Occurrence, isolation and interchain bridges of natural 7-S immunoglobulin M in human serum. Biochim Biophys Acta. 1971 Jun 29;236(3):675–685. [PubMed] [Google Scholar]
- Frank M. M., Humphrey J. H. Spontaneous re-aggregation of IgM subunits and restoration of antibody activity after reduction and alkylation of rabbit anti-Forssman antibody. Immunology. 1969 Aug;17(2):237–247. [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Koshland M. E. Noval subunit in secretory IgA. Nature. 1970 Dec 26;228(5278):1276–1278. doi: 10.1038/2281276a0. [DOI] [PubMed] [Google Scholar]
- Harboe M., Solheim B. G., Deverill J. Individual antigenic specificity of human macroglobulins: Localization of antigenic determinants and their use to demonstrate formation of hybrid molecules after reduction and reassociation. J Exp Med. 1969 Jun 1;129(6):1217–1234. doi: 10.1084/jem.129.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf P. M., Parkhouse R. M., Lennox E. S. Biosynthetic units of an immunoglobulin heavy chain. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2288–2295. doi: 10.1073/pnas.58.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mestecky J., Zikan J., Butler W. T. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971 Mar 19;171(3976):1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- Metzger H. Structure and function of gamma M macroglobulins. Adv Immunol. 1970;12:57–116. doi: 10.1016/s0065-2776(08)60168-6. [DOI] [PubMed] [Google Scholar]
- Miekka S. I., Deutsch H. F. The primary structure of a tetradecapeptide containing the cysteine residue linking gamma M-globulin subunits. J Biol Chem. 1970 Nov 10;245(21):5534–5544. [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Dissociation, reaggregation, and subunit structure studies of some human gamma-M-globulins. J Biol Chem. 1967 Jun 10;242(11):2725–2738. [PubMed] [Google Scholar]
- Tomasi T. B., Jr Production of a noncovalently bonded pentamer of immunoglobulin M: relationship of J chain. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3410–3414. doi: 10.1073/pnas.70.12.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]