Abstract
The effects of Ca++ on primary and secondary immune responses to SRBC in vitro was investigated using the Marbrook technique.
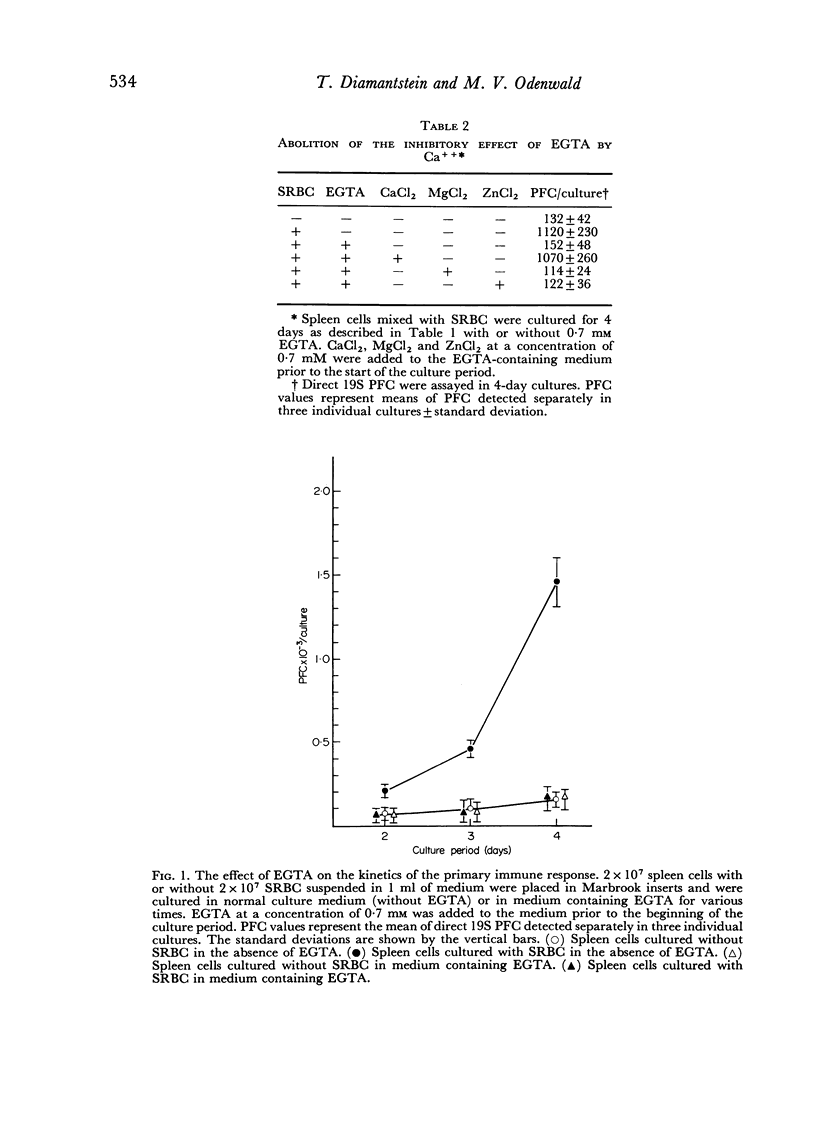
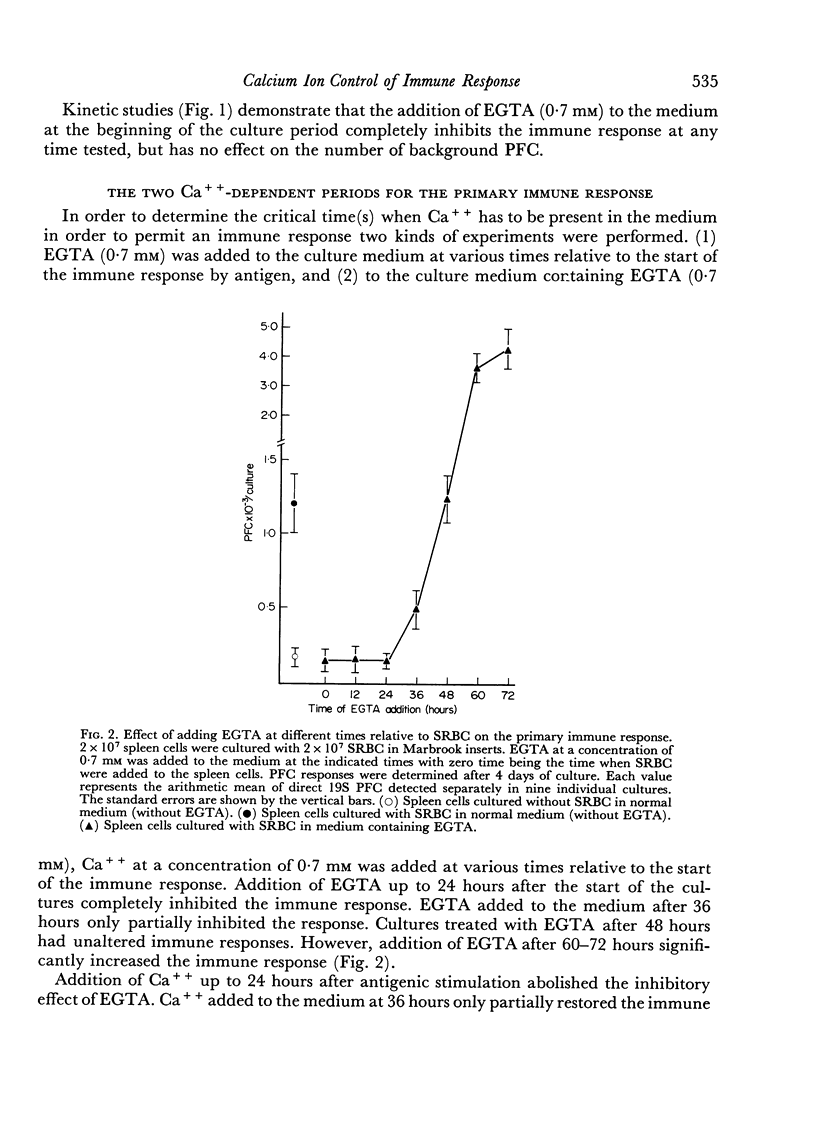
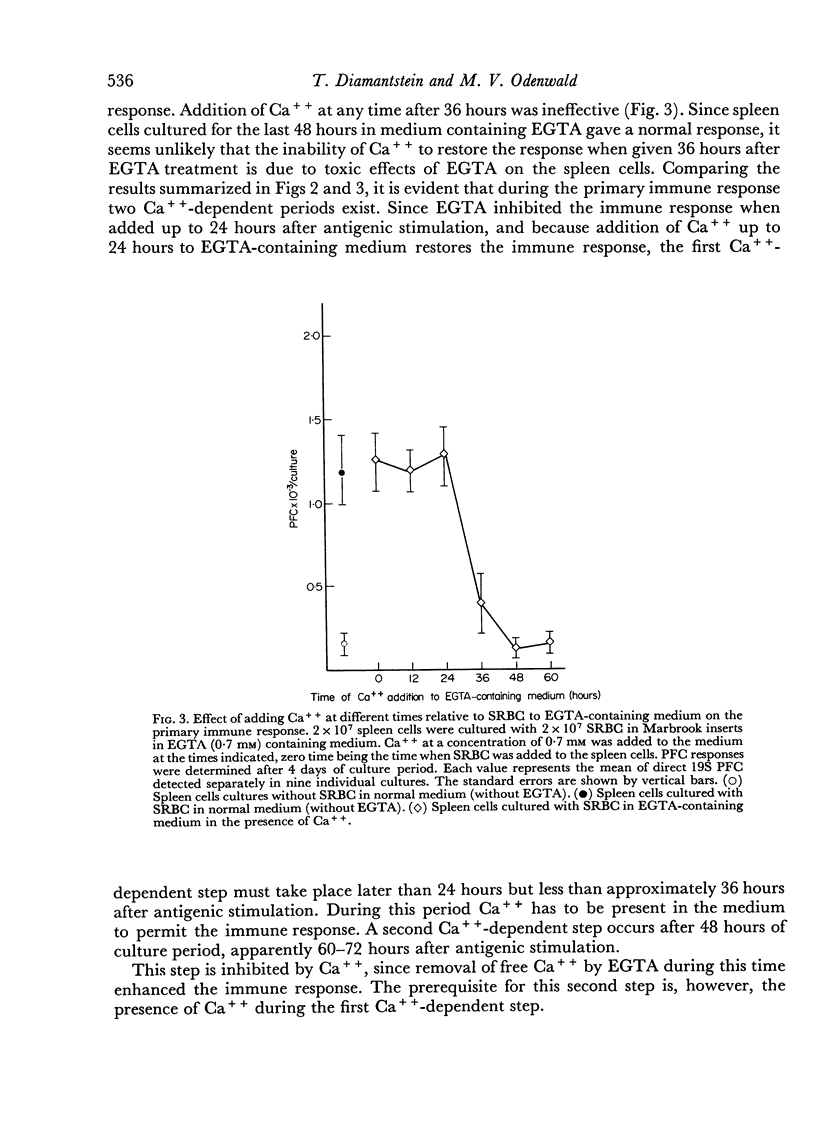
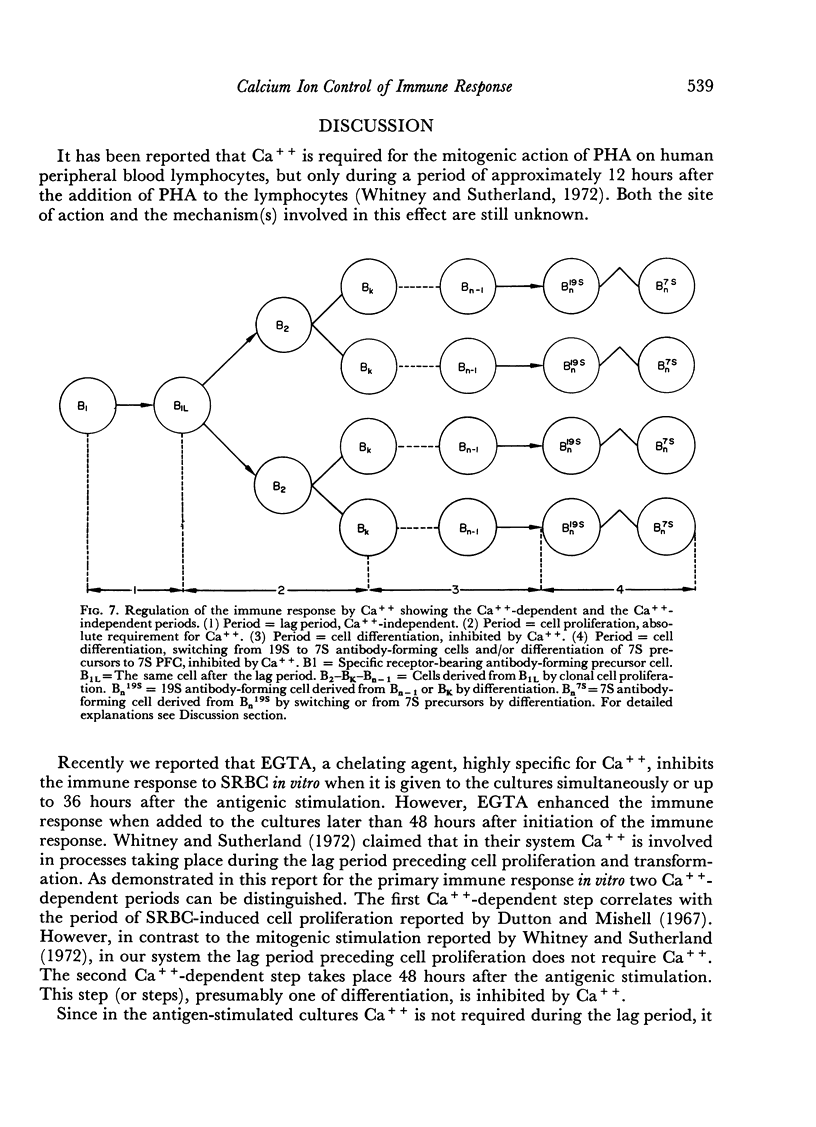
During the primary immune response three periods could be distinguished: first, a Ca++-independent lag period (0–24 hours after antigenic stimulation); second, a period with an absolute requirement for Ca++ (24–36 hours after antigenic stimulation), which is related to a proliferative phase of antigenically stimulated cells; and third, a period (later than 48 hours and up to 72 hours after antigenic stimulation), which is inhibited by Ca++ and which can be enhanced by removing Ca++ from the medium. This third period is related to the differentiation step(s) leading to antibody-forming cells.
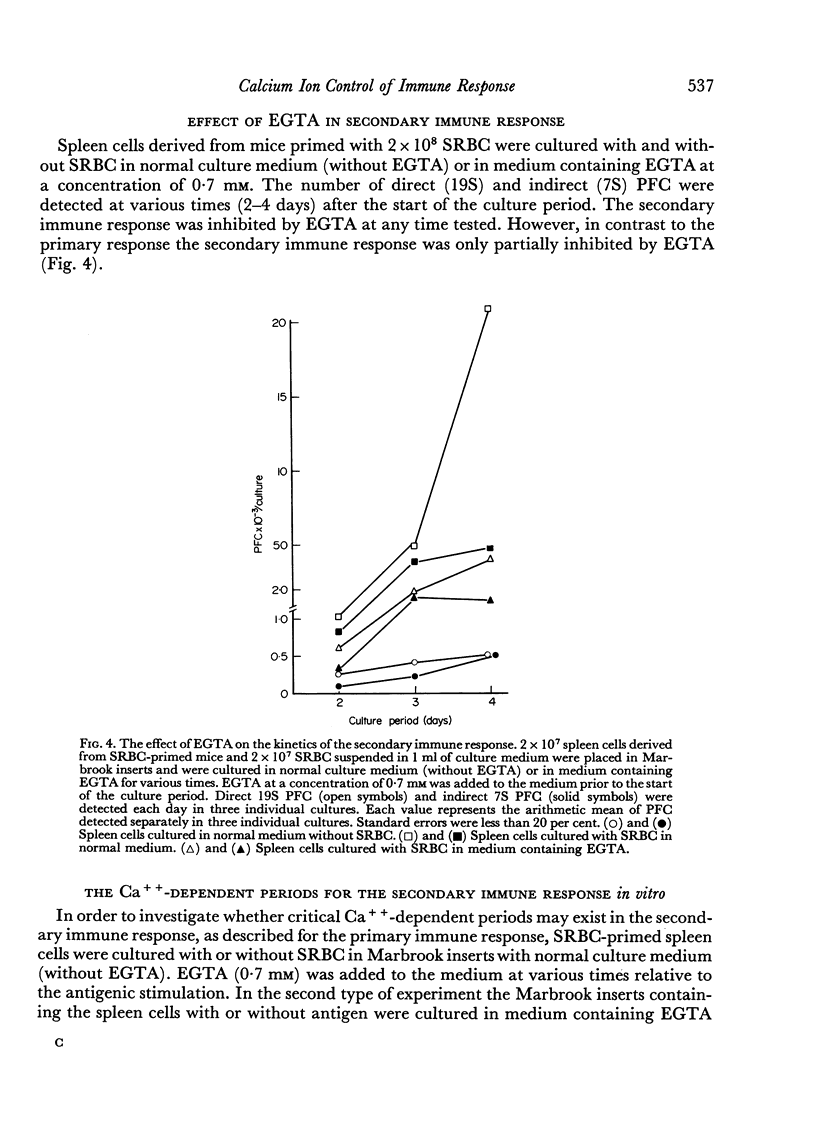
During the secondary immune response only a partial inhibition of immune response was observed after removing Ca++ from the medium at the time of antigenic stimulation.
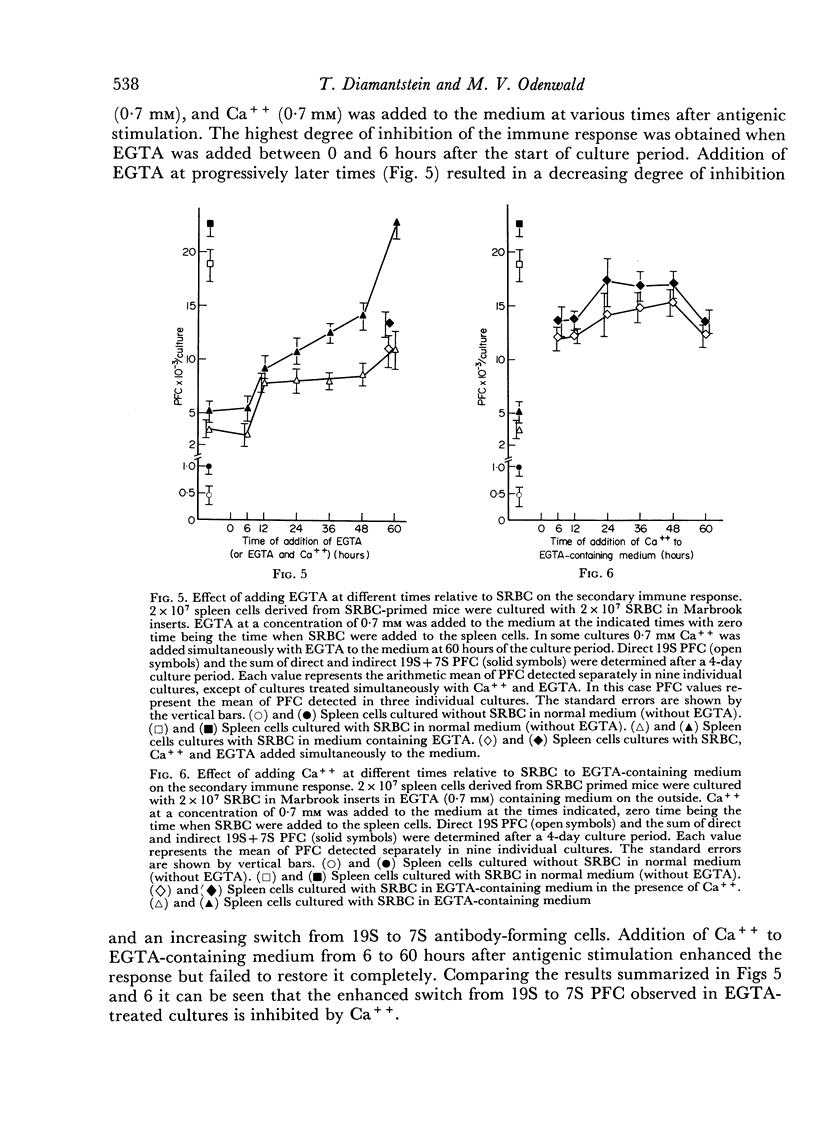
Addition of Ca++ to EGTA-containing culture medium at any time relative to the initiation of the secondary immune response enhanced the response, but, in contrast to its effects on a primary immune response, never completely restored it. Removal of Ca++ later than 6 hours after initiation of the response resulted in a decreased inhibition of the immune response and in an increased switch from 19S to 7S antibody-forming cells. This differentiation step was enhanced by removing Ca++ from the medium and was inhibited by Ca++ added to the medium.
The results suggest that Ca++ controls the mechanisms involved in the antibody formation by an antagonistic action on cell proliferation and cell differentiation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970 Mar;104(3):698–703. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Wagner B., Beyse I., Odenwald M. V., Schulz G. Stimulation of humoral antibody formation by polyanions. I. The effect of polyacrylic acid on the primary immune response in mice immunized with sheep red blood cells. Eur J Immunol. 1971 Nov;1(5):335–340. doi: 10.1002/eji.1830010506. [DOI] [PubMed] [Google Scholar]
- Dukor P., Hartmann K. U. Hypothesis. Bound C3 as the second signal for B-cell activation. Cell Immunol. 1973 Jun;7(3):349–356. doi: 10.1016/0008-8749(73)90199-8. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J. E. Interaction of lymphocytes and phytohaemagglutinin: inhibition by chelating agents. Exp Cell Res. 1971 Sep;68(1):11–16. doi: 10.1016/0014-4827(71)90580-5. [DOI] [PubMed] [Google Scholar]
- Nakamura I., Segal S., Globerson A., Feldman M. DNA replication as a prerequisite for the induction of primary antibody response. Cell Immunol. 1972 Aug;4(4):351–366. doi: 10.1016/0008-8749(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Gillan D. J. The ability of calcium to change cyclic AMP from a stimulator to an inhibitor to thymic lymphoblast proliferation. J Cell Physiol. 1973 Apr;81(2):241–250. doi: 10.1002/jcp.1040810212. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Youdale T., Franks D. J. The roles of calcium and cyclic AMP in the stimulatory action of parathyroid hormone on thymic lymphocyte proliferation. J Cell Physiol. 1971 Dec;78(3):355–368. doi: 10.1002/jcp.1040780305. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]


