Abstract
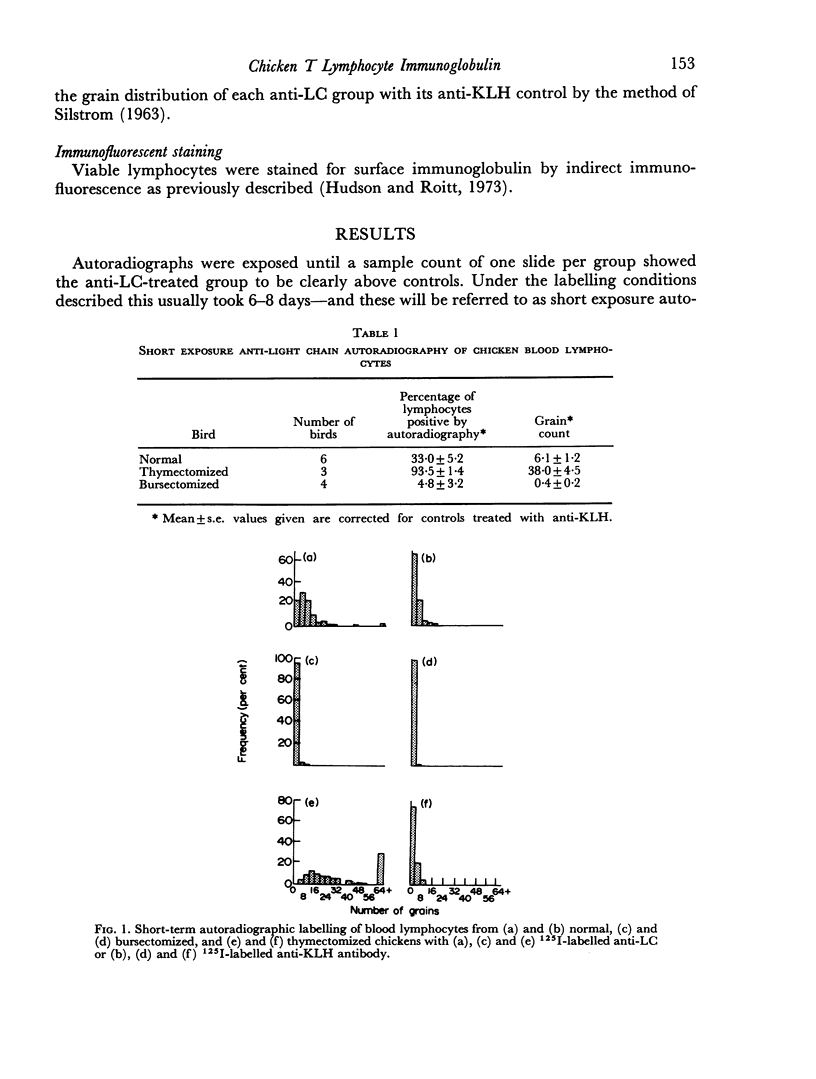
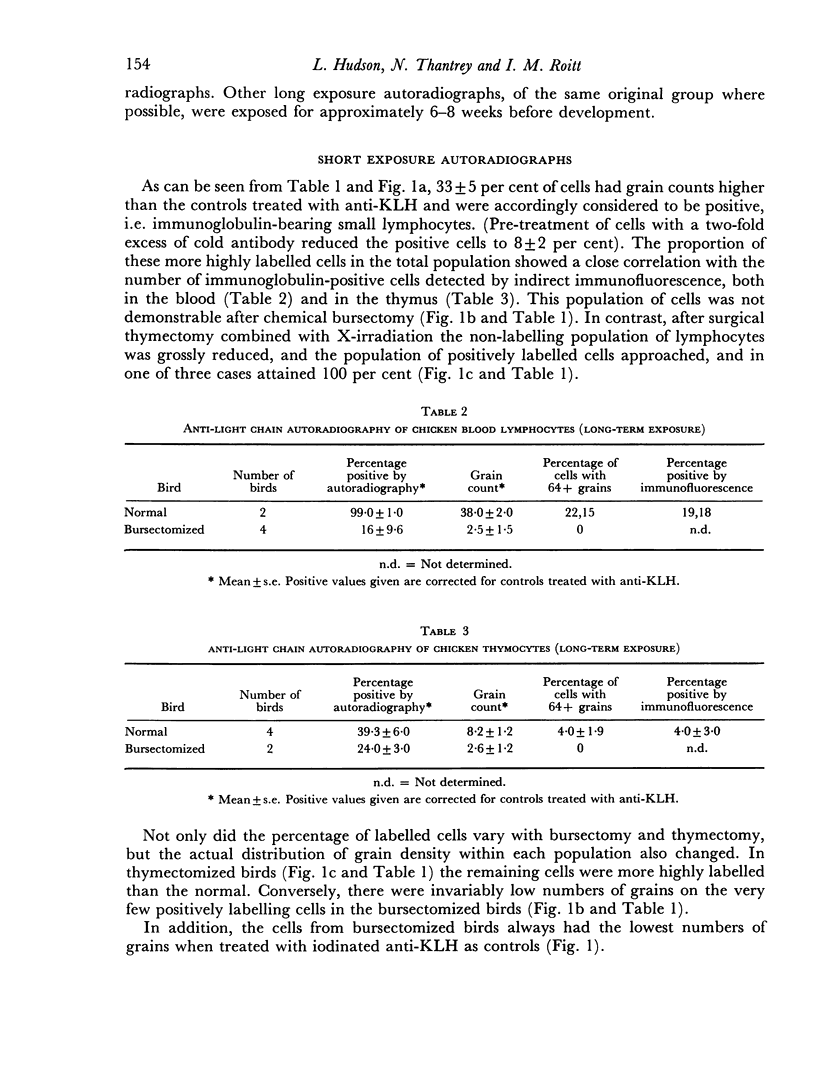
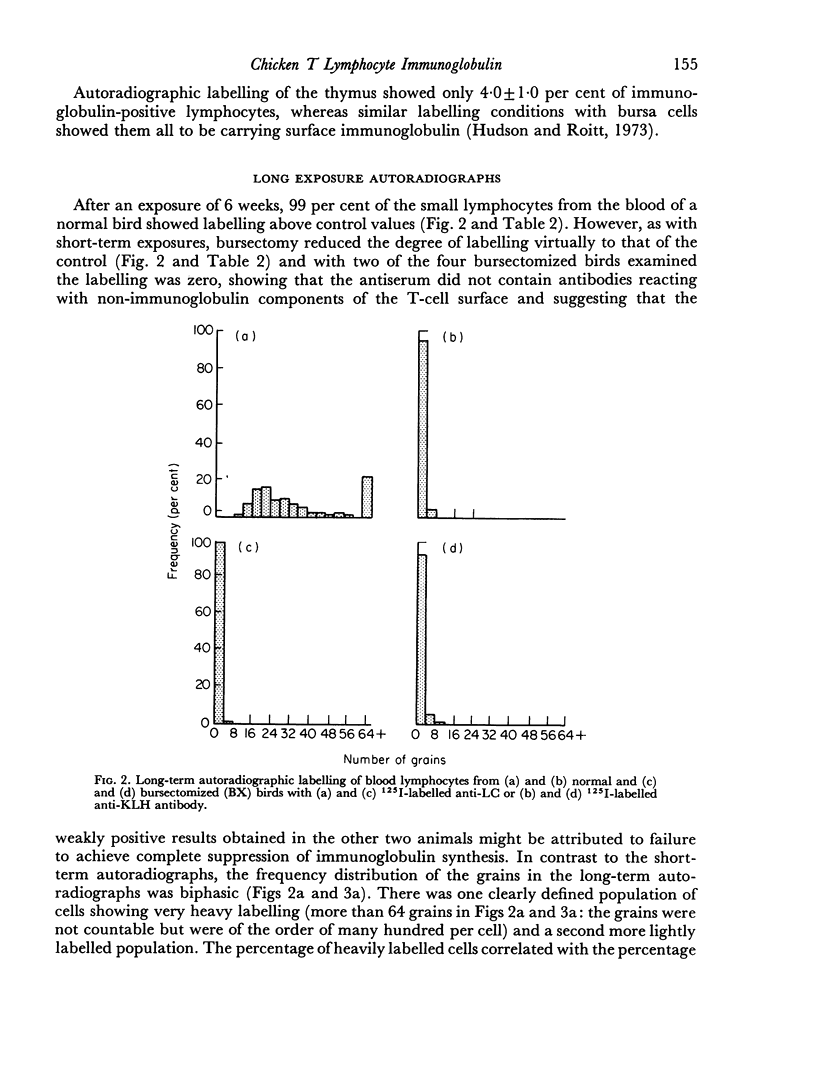
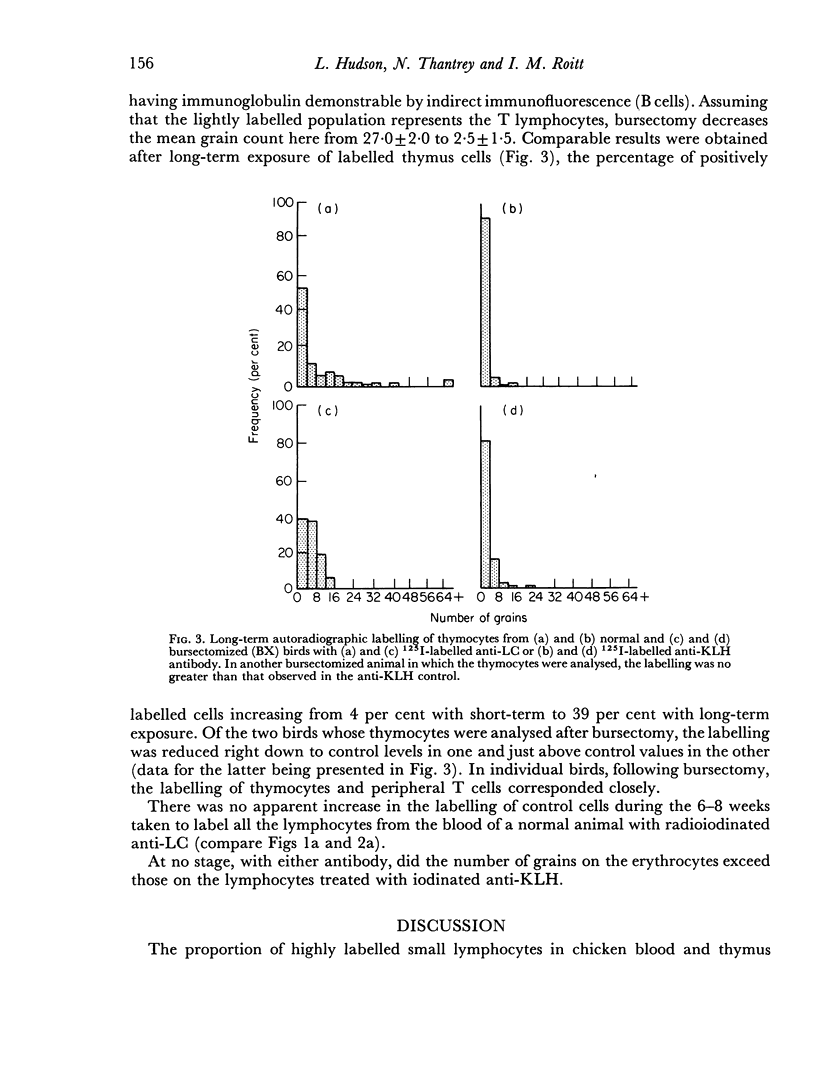
Thirty-three plus or minus 5 per cent of chicken blood lymphocytes were positively labelled by radioiodinated rabbit anti-chicken light chain antibody within 7 days of exposure of autoradiographs. After thymectomy, 93 plus or minus 1 per cent of lymphocytes were positive: conversely, following bursectomy only 5 plus or minus 3 per cent of cells showed positive labelling and then at a very low level. Hence, under these conditions of short-term exposure autoradiography, only B cells carried readily demonstrable immunoglobulin. If the sensitivity of the technique was increased by long-term exposure of the autoradiographs, immunoglobulin was demonstrable on all blood lymphocytes, including the T cells. In addition, 39 plus or minus 6 per cent of thymocytes were shown to carry immunoglobulin by the more sensitive technique. In both cases, however, this T-lymphocyte surface immunoglobulin was reduced, and in some cases absent, after bursectomy. At no stage, with either technique, did the grain count on the erythrocytes reach the control level of lymphocytes treated with 125I-labelled anti-keyhole limpet haemocyanin antibody. It seems likely that T-cell immunoglobulin is not an endogenously synthesized product but is acquired indirectly from the B lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Warner N. L., Sprent J. Surface immunoglobulins on thymus and thymus-derived lymphoid cells. J Exp Med. 1971 Oct 1;134(4):1005–1015. doi: 10.1084/jem.134.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Weiss N., Loor F. Direct evidence for immunoglobulins on the surface of thymus lymphocytes of amphibian larvae. Eur J Immunol. 1972 Aug;2(4):366–370. doi: 10.1002/eji.1830020414. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. The expression of immunoglobulin determinants on the surface of antigen-binding lymphoid cells in mice. I. An analysis of light and heavy chain restrictions on individual cells. Eur J Immunol. 1971 Jun;1(3):186–194. doi: 10.1002/eji.1830010308. [DOI] [PubMed] [Google Scholar]
- Hudson L., Greenberg A. H., Roitt I. M., Bach J. F. The enrichment of T-cell functions in a lymphocyte population by anti-immunoglobulin columns. Scand J Immunol. 1973;2(4):425–431. doi: 10.1111/j.1365-3083.1973.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Hudson L., Roitt I. M. Immunofluorescent detection of surface antigens specific to T and B lymphocytes in the chicken. Eur J Immunol. 1973 Feb;3(2):63–67. doi: 10.1002/eji.1830030202. [DOI] [PubMed] [Google Scholar]
- Hudson L., Sprent J., Miller J. F., Playfair J. H. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 1974 Sep 6;251(5470):60–62. doi: 10.1038/251060a0. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Williams A. F. The origin of cell surface immunoglobulin of marrow-derived and thymus-derived lymphocytes of the rat. J Exp Med. 1974 Mar 1;139(3):479–496. doi: 10.1084/jem.139.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Paul W. E. Hapten-specific augmentation of the anti-idiotype antibody response to hapten-myeloma protein conjugates in mice. Eur J Immunol. 1973 Jun;3(6):340–347. doi: 10.1002/eji.1830030605. [DOI] [PubMed] [Google Scholar]
- Jones G., Torrigiani G., Roitt I. M. Immunoglobulin determinants on mouse lymphocytes. J Immunol. 1971 Jun;106(6):1425–1430. [PubMed] [Google Scholar]
- Kincade P. W., Lawton A. R., Cooper M. D. Restriction of surface immunoglobulin determinants to lymphocytes of the plasma cell line. J Immunol. 1971 May;106(5):1421–1423. [PubMed] [Google Scholar]
- Linna T. J., Frommel D., Good R. A. Effects of early cyclophosphamide treatment on the development of lymphoid organs and immunological functions in the chickens. Int Arch Allergy Appl Immunol. 1972;42(1):20–39. doi: 10.1159/000230590. [DOI] [PubMed] [Google Scholar]
- Marcuson E. C., Roitt I. M. Immunoglobulin allotypic determinants on rabbit lymphocytes. Nature. 1970 Sep 5;227(5262):1051–1053. doi: 10.1038/2271051a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S., Hudson L. Cooperation by mouse T lymphocytes: the role of antibody in T cell specificity. Eur J Immunol. 1974 Jan;4(1):54–56. doi: 10.1002/eji.1830040114. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Grey H. M. Immunoglobulins on the surface of lymphocytes. 3. Bursal origin of surface immunoglobulins on chicken lymphocytes. J Immunol. 1971 May;106(5):1418–1420. [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Cooper M. D. T cells can bind antigen via cytophilic IgM antibody made by B cells. J Immunol. 1973 Jul;111(1):275–277. [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]



