Abstract
Previous studies have shown that lymphocytes from patients with chronic lymphocytic leukaemia have a diminished response to mitogens which stimulate T cells. Chronic lymphocytic leukaemia is most often a disease of accumulating B cells so that T lymphocytes are diluted by large numbers of leukaemic cells. Direct comparison with the responses of normal lymphocytes to mitogenic stimulation is therefore suspect. To circumvent this difficulty, a method of isolating T cells from normal individuals and patients with chronic lymphocytic leukaemia was developed. Lymphocytes containing an average of 16.1 per cent B cells from normal individuals were applied to IgG-anti-IgG-coated Degalan bead columns and held at 4 degrees for 2 hours. The eluted cells contained less than 2 per cent B cells. When chronic lymphocytic leukaemic lymphocytes, containing an average of 68.6 per cent B cells, were applied to IgG-anti-IgG columns, the eluted cells contained 36.4 per cent B cells. To improve the purification of T lymphocytes, columns of uncoated Degalan beads were used to remove non-specifically adherent cells. Eluted lymphocytes were then applied to IgG-anti-IgG columns. This resulted in the recovery of purified populations of T cells with less than 2 per cent contamination with B cells. Patients with chronic lymphocytic leukaemia were found to have lymphocytes with either a normal density or a low density of surface immunoglobulins. B cells were successfully removed from lymphocyte suspensions in all cases of chronic lymphocytic leukaemia with a normal density of lymphocyte surface immunoglobulins. In the three cases of chronic lymphocytic leukaemia with low density surface immunoglobulins, separation by this method was unsuccessful. However, an enriched T-cell population was obtained when leukaemic lymphocytes which had lost all detectable surface immunoglobulins were passed through a column coated with heat-aggregated IgG.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. B. A physical adherence column method for the preparation of T and B lymphocytes from normal mouse spleen. Cell Immunol. 1973 Sep;8(3):356–371. doi: 10.1016/0008-8749(73)90126-3. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Bloch K. J. Immunoglobulins on the surface of neoplastic lymphocytes. N Engl J Med. 1972 Aug 10;287(6):272–276. doi: 10.1056/NEJM197208102870603. [DOI] [PubMed] [Google Scholar]
- Basten A., Sprent J., Miller J. F. Receptor for antibody-antigen complexes used to separate T cells from B cells. Nat New Biol. 1972 Feb 9;235(58):178–180. doi: 10.1038/newbio235178a0. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Weiss D. W., Sulitzeanu D., Kedar E., Izak G., Cohen I., Eyal O. Antigenic changes on the surface of lymphocytes from patients with chronic lymphocytic leukemia. Cancer Res. 1972 Jul;32(7):1375–1383. [PubMed] [Google Scholar]
- Bouroncle B. A., Clausen K. P., Aschenbrand J. F. Studies of the delayed response of phytohemagglutinin (PHA) stimulated lymphocytes in 25 chronic lymphatic leukemia patients before and during therapy. Blood. 1969 Aug;34(2):166–178. [PubMed] [Google Scholar]
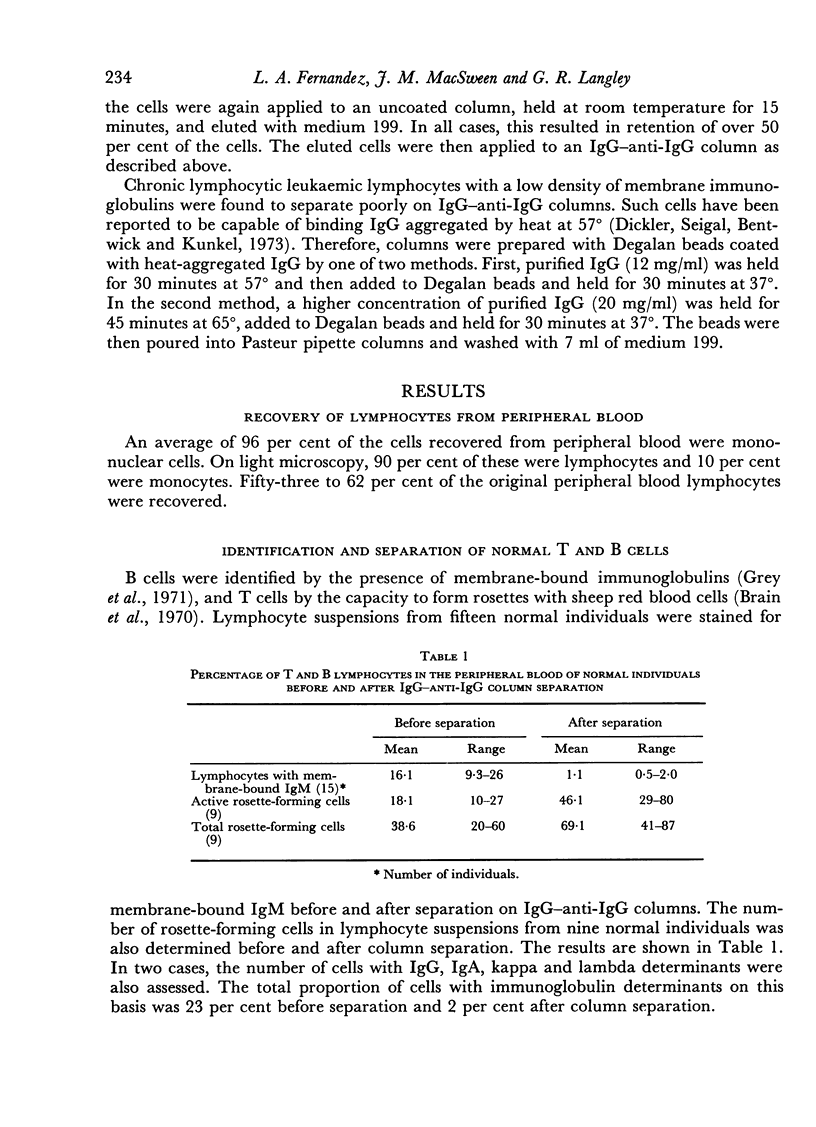
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Coates A. S., Lennon V. A. Lymphocytes binding basic protein of myelin; cytophilic serum antibody and effect of adjuvant. Immunology. 1973 Mar;24(3):425–434. [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Siegal F. P., Bentwich Z. H., Kunkel H. G. Lymphocyte binding of aggregated IgG and surface Ig staining in chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 May;14(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Dán P., Kulcsár G., Sallay K., Nász I. Human lymphocyte transformation with virus antigens. Blut. 1971 Apr;22(4):211–215. doi: 10.1007/BF01633616. [DOI] [PubMed] [Google Scholar]
- Fridman W. H., Kourilsky F. M. Stimulation of lymphocytes by autologous leukaemic cells in acute leukaemia. Nature. 1969 Oct 18;224(5216):277–279. doi: 10.1038/224277a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Pierce G. E., Yang J. P. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968 Dec 28;220(5174):1352–1354. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. S., Fudenberg H. H., Hopper J. E., Wilson S. K., Nisonoff A. Immunofluorescent evidence for cellular control of synthesis of variable regions of light and heavy chains of immunoglobulins G and M by the same gene. Proc Natl Acad Sci U S A. 1971 Jan;68(1):169–171. doi: 10.1073/pnas.68.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER D. G., LIZARDO J. G., SNYDERMAN R. K. Homologous and heterologous skin transplantation in patients with lymphomatous disease. J Natl Cancer Inst. 1961 Mar;26:569–583. [PubMed] [Google Scholar]
- MacSween J. M., Langley G. R. Intraleucocytic immunoglobulin in eosiniophilia in man. Immunology. 1971 Jul;21(1):61–68. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin H., Wetherly-Mein G., Pitcher C., Hobbs J. R. Non-immunoglobulin-bearing 'B' lymphocytes in chronic lymphatic leukaemia? Br J Haematol. 1973 Jul;25(1):7–14. doi: 10.1111/j.1365-2141.1973.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972 Dec;40(6):777–794. [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Herberman R., Frank M. M., Green I. Receptors for complement and immunoglobulin on human leukemic cells and human lymphoblastoid cell lines. J Clin Invest. 1972 Aug;51(8):1933–1938. doi: 10.1172/JCI106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Byrd W., Williams N., Brunner K. T., Cerottini J. C. The separation of different cell classes from lymphoid organs. The relationship between the adherence properties and the buoyant density of sub-populations of "B" and "T" lymphocytes. Aust J Exp Biol Med Sci. 1972 Jun;50(3):323–336. doi: 10.1038/icb.1972.26. [DOI] [PubMed] [Google Scholar]
- Sjögren H. O., Hellström I., Bansal S. C., Warner G. A., Hellström K. E. Elution of "blocking factors" from human tumors, capable of abrogating tumor-cell destruction by specifically immune lymphocytes. Int J Cancer. 1972 Mar 15;9(2):274–283. doi: 10.1002/ijc.2910090205. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Cowling D. C., Barker C. R. Response of lymphocytes in chronic lymphocytic leukaemia to plant mitogens. Lancet. 1972 Jan 29;1(7744):229–233. doi: 10.1016/s0140-6736(72)90624-1. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Hurdle A. D. Surface immunoglobulins on lymphocytes in chronic lymphocytic leukaemia and lymphosarcoma. Br J Haematol. 1973 May;24(5):563–569. doi: 10.1111/j.1365-2141.1973.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]



