Abstract
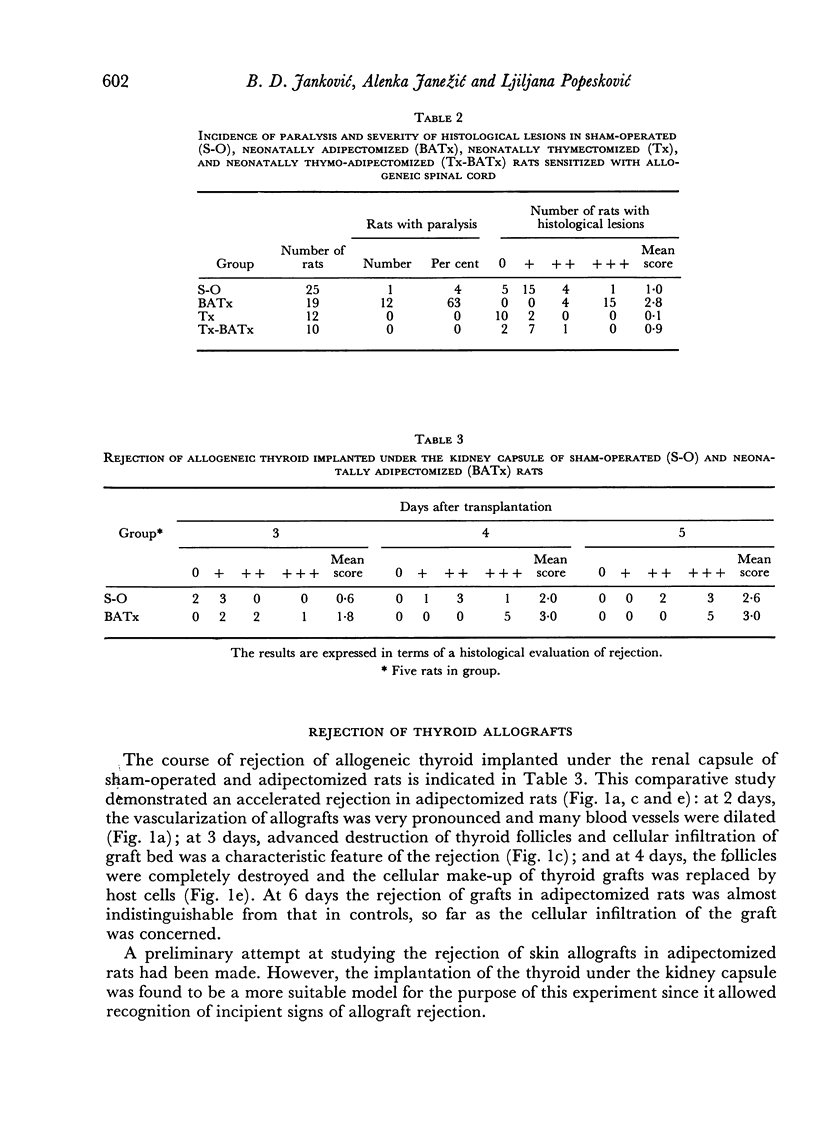
This work concerns the involvement of brown adipose tissue in the immune system ofthe rat. Wistar rats were thymectomized, adipectomized (surgical extirpation of the interscapular brown adipose tissue), thymectomized and adipectomized, and sham-operatedat birth, only 8-week-old females being employed in the experiment. The production of antibody to bovine werum albumin (BSA) and sheep red blood cells (SRBC), delayed skin reactions to BSA, rejection of thyroid allograft implanted under the kidney capsule, and development of experimental allergic encephalomyelitis were investigated. Neonatal adipectomy did not affect the production of anti-BSA and anti-SRBC antibodies. On the other hand, delayed skin reactions to BSA, rejection of thyroid allograft, and incidence and severity of allergic encephalomyelitis were much more pronounced in adipectomized animals. It has been postulated that the immune function of brown adipose tissue is an expression of the secretory activity of the tissue. Since the immunosuppressive effect of neonatal thymectomy on demyelinating disease was neutralized by neonatal adipectomy, and vice versa, and since thymectomy rendered ineffective the immunopotentiating influence of adipectomy on this disease, as demonstrated in thymo-adipectomized rats, it was concluded that the brown adipose tissue is a naturalantagonist of the thymus in cell-mediated immunity. This paper also describes the extra thymuses which were situated in the vicinity of the thyroid and parathyroid lobes of23.2 per cent of rats.
Full text
PDF











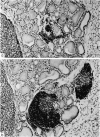
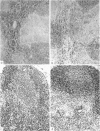
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWKINS M. J., HULL D. BROWN ADIPOSE TISSUE AND THE RESPONSE OF NEW-BORN RABBITS TO COLD. J Physiol. 1964 Aug;172:216–238. doi: 10.1113/jphysiol.1964.sp007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Simpson B. A., Richerson H. B., Leskowitz S., Karnovsky M. J. Cutaneous basophil hypersensitivity. II. A light and electron microscopic description. J Exp Med. 1970 Sep 1;132(3):558–582. doi: 10.1084/jem.132.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANKOVIC B. D., WAKSMAN B. H., ARNASON B. G. Role of the thymus in immune ractions in rats. I. The immunologic response to bovine serum albumin (antibody formation, Arthus reactivity, and delayed hypersensitivity) in rats thymectomized or splenectomized at various times after birth. J Exp Med. 1962 Aug 1;116:159–176. doi: 10.1084/jem.116.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAROSLOW B. N., SMITH D. E. Antigen disappearance in hibernating ground squirrels. Science. 1961 Sep 15;134(3481):734–735. doi: 10.1126/science.134.3481.734. [DOI] [PubMed] [Google Scholar]
- Sidky Y. A., Daggett L. R., Auerbach R. Brown fat: its possible role in immunosuppression during hibernation. Proc Soc Exp Biol Med. 1969 Nov;132(2):760–763. doi: 10.3181/00379727-132-34305. [DOI] [PubMed] [Google Scholar]
- Sidky Y. A., Hayward J. S., Ruth R. F. Seasonal variations of the immune response of ground squirrels kept at 22-24 degrees C. Can J Physiol Pharmacol. 1972 Mar;50(3):203–206. doi: 10.1139/y72-031. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., ARNASON B. G., JANKOVIC B. D. Role of the thymus in immune reactions in rats. III. Changes in the lymphoid organs of thymectomized rats. J Exp Med. 1962 Aug 1;116:187–206. doi: 10.1084/jem.116.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]