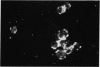
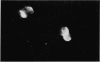
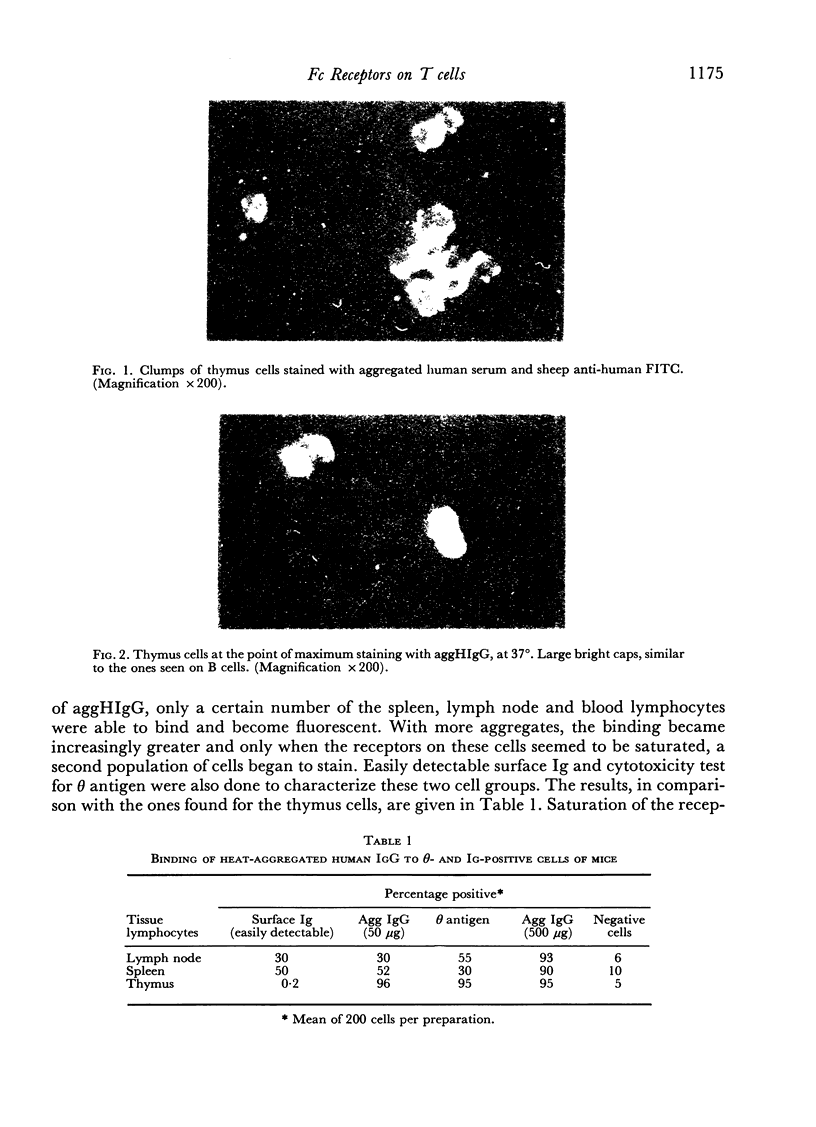
Abstract
Normal thymus lymphocytes and T cells of mice have the ability to bind heat-aggregated IgG of human origin (aggHIgG), as shown by indirect immunofluorescence. At 4 degrees, the cells bind aggHIgG with an irregular speckled appearance; at 37 degrees, the aggregates are incorporated into the surface membrane, inducing rearrangement of the receptors and capping. At the point of maximum binding capacity, thymocytes show a fairly homogeneous fluorescence pattern, whereas T cells show a heterogeneous appearance. Aggregated pure human Fc fragments, but not Fab fragments, retained not only the binding capacity of the complete molecule but also the ability to induce cap formation on the surface of thymusceds, at 37 degrees.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Sprent J., Miller J. F. Receptor for antibody-antigen complexes used to separate T cells from B cells. Nat New Biol. 1972 Feb 9;235(58):178–180. doi: 10.1038/newbio235178a0. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Cogen R. B. Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J Exp Med. 1973 Jul 1;138(1):163–175. doi: 10.1084/jem.138.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. II. Nature of the immunoglobulin determinants. Immunology. 1972 Jun;22(6):967–980. [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz C., Hahn Y. Cell-surface immunoglobulin human thymus cells and its biosynthesis in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3716–3720. doi: 10.1073/pnas.70.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas F., Lee S. T., Orr K. B., Israels L. G. A receptor for Fc on mouse B-lymphocytes. J Immunol. 1972 May;108(5):1319–1327. [PubMed] [Google Scholar]
- Roelants G. E., Rydén A., Hägg L. B., Loor F. Active synthesis of immunoglobulin receptors for antigen by T lymphocytes. Nature. 1974 Jan 11;247(5436):106–108. doi: 10.1038/247106a0. [DOI] [PubMed] [Google Scholar]
- Santana V., Wedderburn N., Turk J. L. Demonstration of immunoglobulin on the surface of thymus lymphocytes. Immunology. 1974 Jul;27(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Rosenstreich D. L. Binding of aggregated gamma-globulin to activated T lymphocytes in the guinea pig. J Exp Med. 1974 Apr 1;139(4):1002–1012. doi: 10.1084/jem.139.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]