Abstract
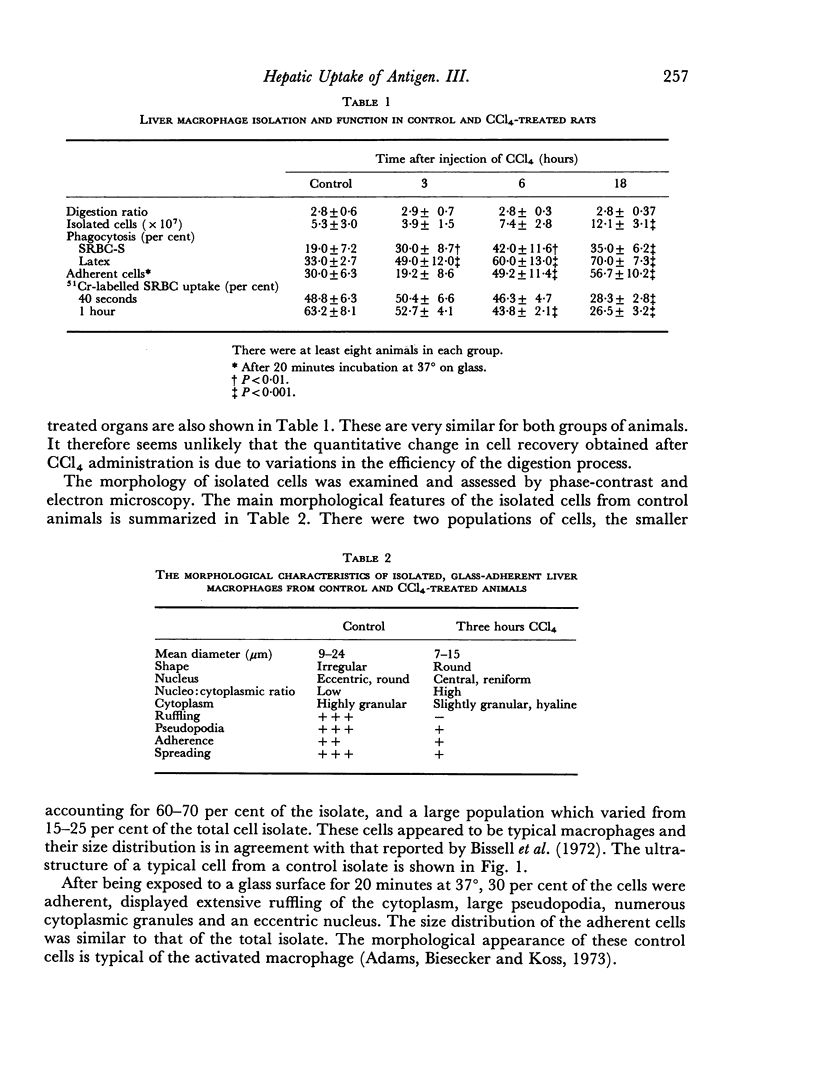
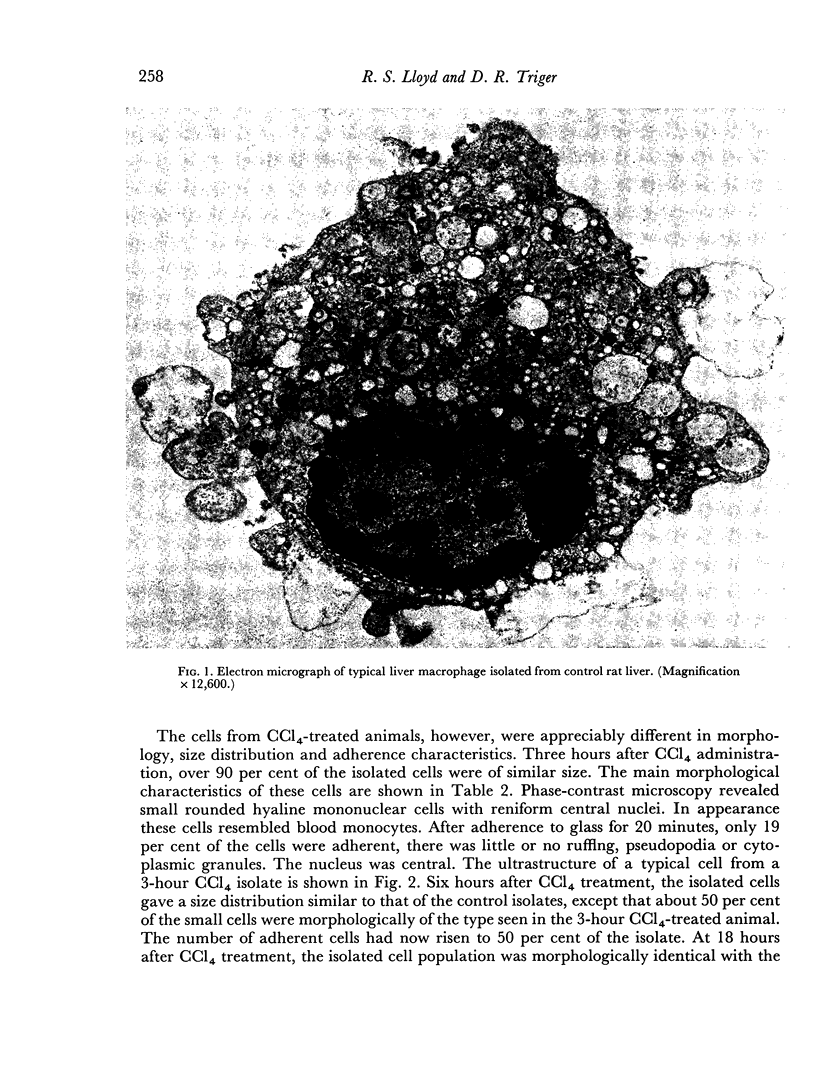
A technique of pronase digestion followed by density gradient centrifugation was used to study the morphology and phagocytic function of isolated rat liver macrophages in the normal rat and at varying times after the in vivo administration of carbon tetrachloride. Administration of the hepatotoxin results in a transient fall in the number of isolated macrophages. This deficit is corrected by a rapid influx of mononuclear cells from elsewhere in the animal which quickly differentiate into liver macrophages. Despite changes in the morphology of isolated macrophages, no evidence was found to suggest in vitro functional impairment of these cells. In vivo studies of intrahepatic shunting showed that this became a significant phenomenon 6 hours after the administration of the hepatotoxin. The enhanced antibody response to sheep red cells which occurs after carbon tetrachloride administration appears to be due to a series of events in which the decrease in the number of liver macrophages is an early significant factor, while intrahepatic shunting is a major contributing factor at a later time.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Biesecker J. L., Koss L. G. The activation of mononuclear phagocytes in vitro: immunologically mediated enhancement. J Reticuloendothel Soc. 1973 Dec;14(6):550–570. [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Liver sinusoidal cells. Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972 Jul;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boak J. L., Christie G. H., Ford W. L., Howard J. G. Pathways in the development of liver macrophages: alternative precursors contained in populations of lymphocytes and bone-marrow cells. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):307–327. doi: 10.1098/rspb.1968.0013. [DOI] [PubMed] [Google Scholar]
- Cantor H. M., Dumont A. E. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967 Aug 12;215(5102):744–745. doi: 10.1038/215744a0. [DOI] [PubMed] [Google Scholar]
- Curley D. M., Adler L. T., Fishman M. Studies on antibody induction in vitro. II. Cellular requirements for a primary response to soluble T2 antigens by rabbit cell cultures. Immunology. 1974 Oct;27(4):553–562. [PMC free article] [PubMed] [Google Scholar]
- Inchley C. J. The actvity of mouse Kupffer cells following intravenous injection of T4 bacteriophage. Clin Exp Immunol. 1969 Jul;5(1):173–187. [PMC free article] [PubMed] [Google Scholar]
- PARONETTO F., POPPER H. ENHANCED ANTIBODY FORMATION IN EXPERIMENTAL ACUTE AND CHRONIC LIVER INJURY PRODUCED BY CARBON TETRACHLORIDE OR ALLYL ALCOHOL. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1060–1064. doi: 10.3181/00379727-116-29451. [DOI] [PubMed] [Google Scholar]
- Souhami R. L. The effect of colloidal carbon on the organ distribution of sheep red cells and the immune response. Immunology. 1972 Apr;22(4):685–694. [PMC free article] [PubMed] [Google Scholar]
- Triger D. R., Cynamon M. H., Wright R. Studies on hepatic uptake of antigen. I. Comparison of inferior vena cava and portal vein routes of immunization. Immunology. 1973 Dec;25(6):941–950. [PMC free article] [PubMed] [Google Scholar]
- Triger D. R., Wright R. Hyperglobulinaemia in liver disease. Lancet. 1973 Jun 30;1(7818):1494–1496. doi: 10.1016/s0140-6736(73)91827-8. [DOI] [PubMed] [Google Scholar]
- Triger D. R., Wright R. Studies on hepatic uptake of antigen. II. The effect of hepatotoxins on the immune response. Immunology. 1973 Dec;25(6):951–956. [PMC free article] [PubMed] [Google Scholar]
- Warr G. W., Sljivić V. S. Origin and division of liver macrophages during stimulation of the mononuclear phagocyte system. Cell Tissue Kinet. 1974 Nov;7(6):559–565. doi: 10.1111/j.1365-2184.1974.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res. 1972 Mar;38(5):528–562. doi: 10.1016/0022-5320(72)90089-5. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]