Abstract
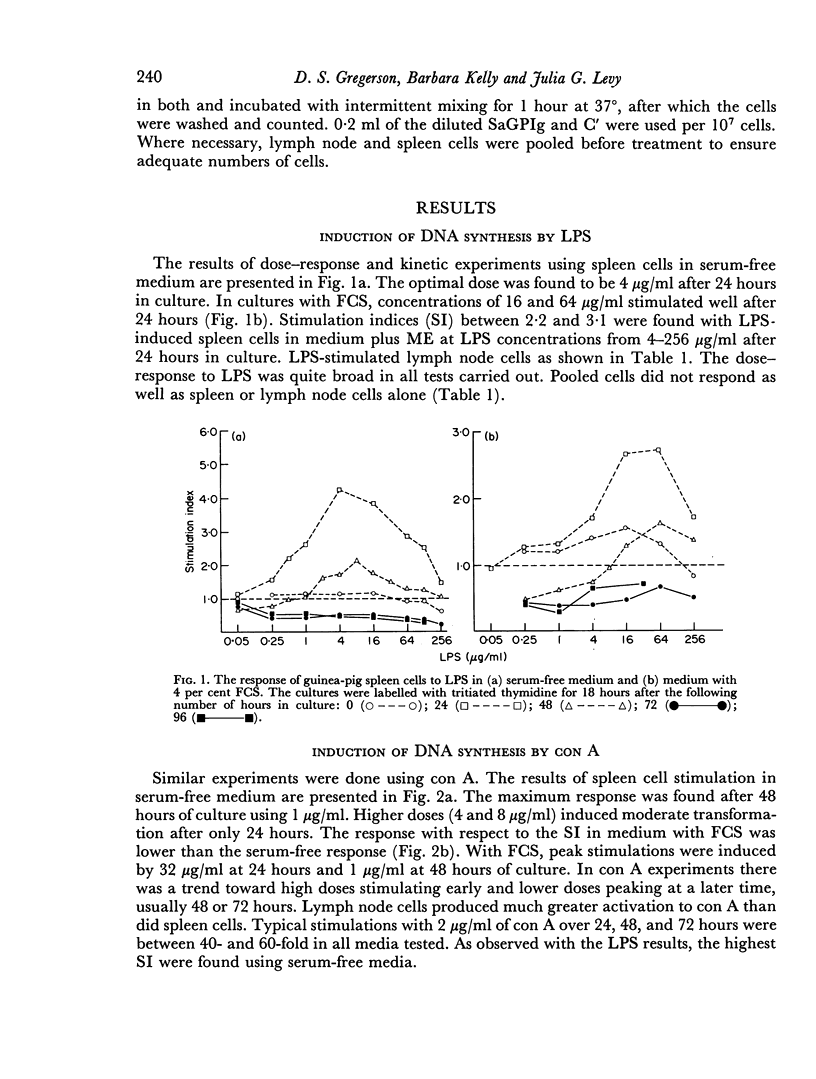
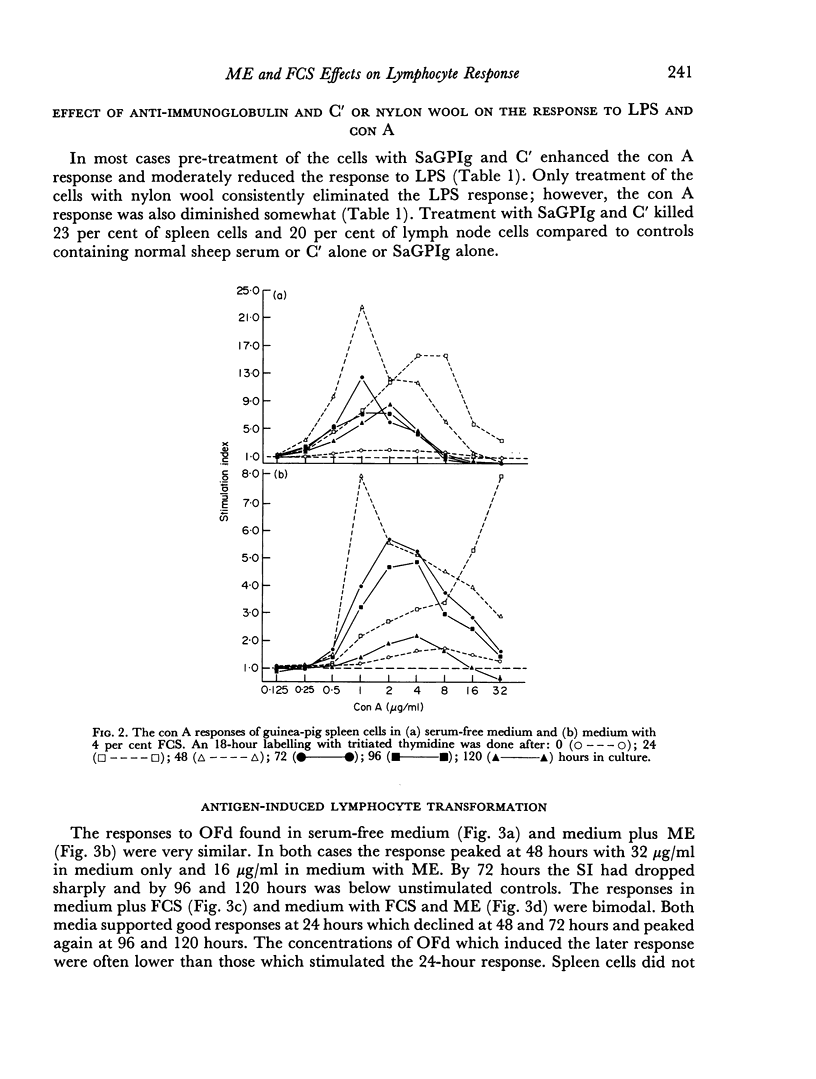
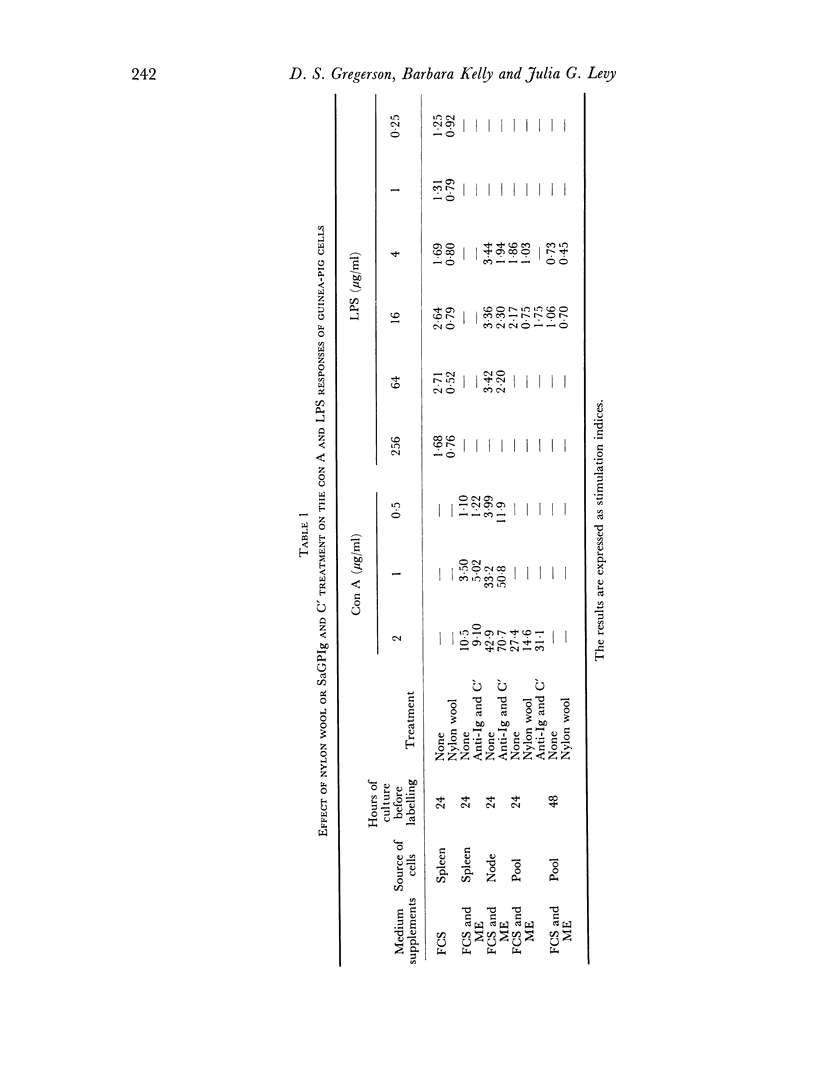
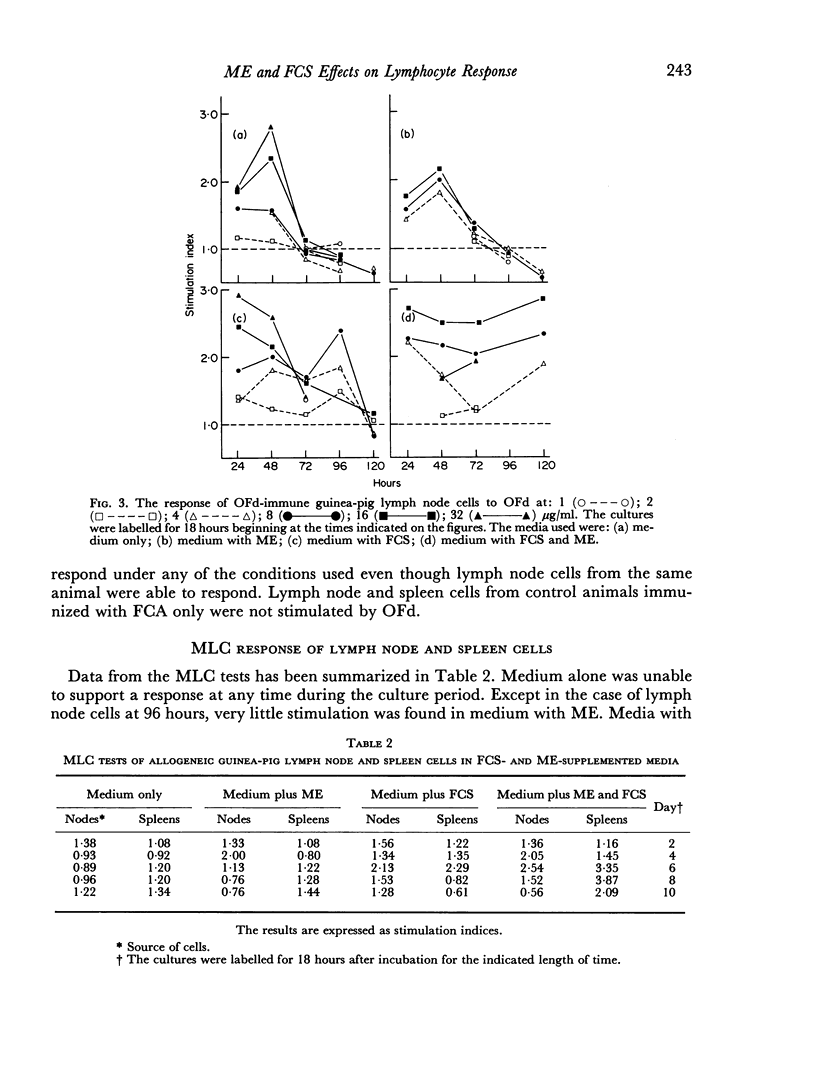
The ability of guinea-pig spleen and lymph node cells to undergo a proliferative response in vitro in the presence of mitogens (concanavalin A and lipopolysaccharide), a specific antigen (oxidized ferredoxin), and allogeneic cells was assessed under a variety of conditions. Time and dose dependency of the responses was measured in RPMI 1640, RPMI 1640 plus mercaptoethanol (ME), RPMI 1640 plus foetal calf serum (FCS), and RPMI 1640 with ME and FCS. Mitogen responses were also measured after treatment of the cells with sheep antiguinea pig immunoglobulin (SaGPIg) and complement (C') or after passage through nylon wool columns. Lipopolysaccharide (LPS) stimulated the cells under all media conditions over a wide range of concentrations but over a narrow time period. Nylon wool treatment of the cells eliminated the LPS response while SaGPIg and C' reduced it. Concanavalin A (con A) stimulated the cells under all test conditions and demonstrated a dose-time interrelationship in terms of maximum response. Pre-treatment of cells with SaGPIg and C' enhanced the response to con A while nylon wool fractionation diminished it somewhat. Only lymph node cells responded in vitro to oxidized ferredoxin (OFd). In serum-free media the OFd responses were maximal at 48 hours whereas in media containing FCS proliferative responses were supported for a prolonged period and appeared to be bimodal. Except for an early response with RPMI 1640 and ME, only media containing FCS supported stimulation in the mixed leucocyte culture.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Bevan M. J., Epstein R., Cohn M. The effect of 2-mercaptoethanol on murine mixed lymphocyte cultures. J Exp Med. 1974 Apr 1;139(4):1025–1030. doi: 10.1084/jem.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein G. J., Harrison M. R., Green I. Demonstration of proliferation by bone marrow-derived lymphocytes of guinea pigs, mice and rabbits in response to mitogen stimulation in vitro. J Immunol. 1973 May;110(5):1334–1339. [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Enhancement by reducing agents of the transformation of human and rabbit peripheral lymphocytes. J Immunol. 1970 Oct;105(4):1043–1045. [PubMed] [Google Scholar]
- Gmelig Meyling F. H., Kooy-Blok L., Ballieux R. E. Complement-dependent cytolysis of human B lymphocytes with anti-light chain antisera. Eur J Immunol. 1974 May;4(5):332–337. doi: 10.1002/eji.1830040504. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Click R. E. Immune responses in vitro. V. Role of mercaptoethanol in the mixed-leukocyte reaction. Cell Immunol. 1972 Nov;5(3):410–418. doi: 10.1016/0008-8749(72)90067-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Oppenheim J. J. Stimulation of chicken lymphocytes in a serum-free medium. Cell Immunol. 1972 Apr;3(4):695–699. doi: 10.1016/0008-8749(72)90131-1. [DOI] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B., Levy J. G., Nitz R. M. Synthesis of haptenic C-terminal octapeptides of two cross-reacting bacterial ferredoxin molecules. Biochemistry. 1970 Apr 14;9(8):1839–1844. doi: 10.1021/bi00810a026. [DOI] [PubMed] [Google Scholar]
- Mugraby L., Gery I., Sulitzeanu D. The participation of thymus-derived and of bone marrow-derived lymphocytes of sensitized mice, in the proliferative response to specific antigen, in vitro. Eur J Immunol. 1974 Jun;4(6):402–405. doi: 10.1002/eji.1830040603. [DOI] [PubMed] [Google Scholar]
- Möller G., Andersson J., Pohlit H., Sjöberg O. Quantitation of the number of mitogen molecules activating DNA synthesis in T and B lymphocytes. Clin Exp Immunol. 1973 Jan;13(1):89–99. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman G. B., Strong D. M., Ahmed A., Green S. S., Sell K. W., Hartzman R. J., Bach F. H. Human mixed lymphocyte cultures. Evaluation of a microculture technique utilizing the Multiple Automated Sample Harvester (MASH). Clin Exp Immunol. 1973 Oct;15(2):289–302. [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L. Culture of mouse lymphoid cells in serum-free medium. J Immunol Methods. 1972 Jan;1(2):199–202. doi: 10.1016/0022-1759(72)90046-4. [DOI] [PubMed] [Google Scholar]
- Weber W. T. Direct evidence for the response of B and T cells to pokeweed mitogen. Cell Immunol. 1973 Dec;9(3):482–487. doi: 10.1016/0008-8749(73)90064-6. [DOI] [PubMed] [Google Scholar]



