Abstract
The cellular activity of circulating lymphocytes and lymphocytes isolated from the infected kidney of animals with experimental haematogenous pyelonephritis was evaluated. The incorporation of [3H-methyl]thymidine into DNA by lymphocytes was studied with mitogens such as phytohaemagglutinin (PHA), pokeweek mitogen (PWM) and goat anti-rabbit IgG (GARIG). Lymphocytes from infected kidney had a high baseline DNA synthesis compared to circulating lymphocytes from days 5 to 27 of infection. Infected kidney lymphocytes failed to respond to PHA, PWM, or GARIG, whereas circulating lymphocytes did respond to these mitogens. Uropod-bearing lymphocytes, which were shown to be T lymphocytes, were present from days 5 to 77 of infection. B lymphocytes, as determined by surface immunofluorescent technique, were present by day 12, coincident with the onset of local synthesis of antibody. These studies reveal that in pyelonephritis, the cellular response goes through sequential changes and indicate a dynamic interrelationship between T and B lymphocytes at an infected site.
Full text
PDF


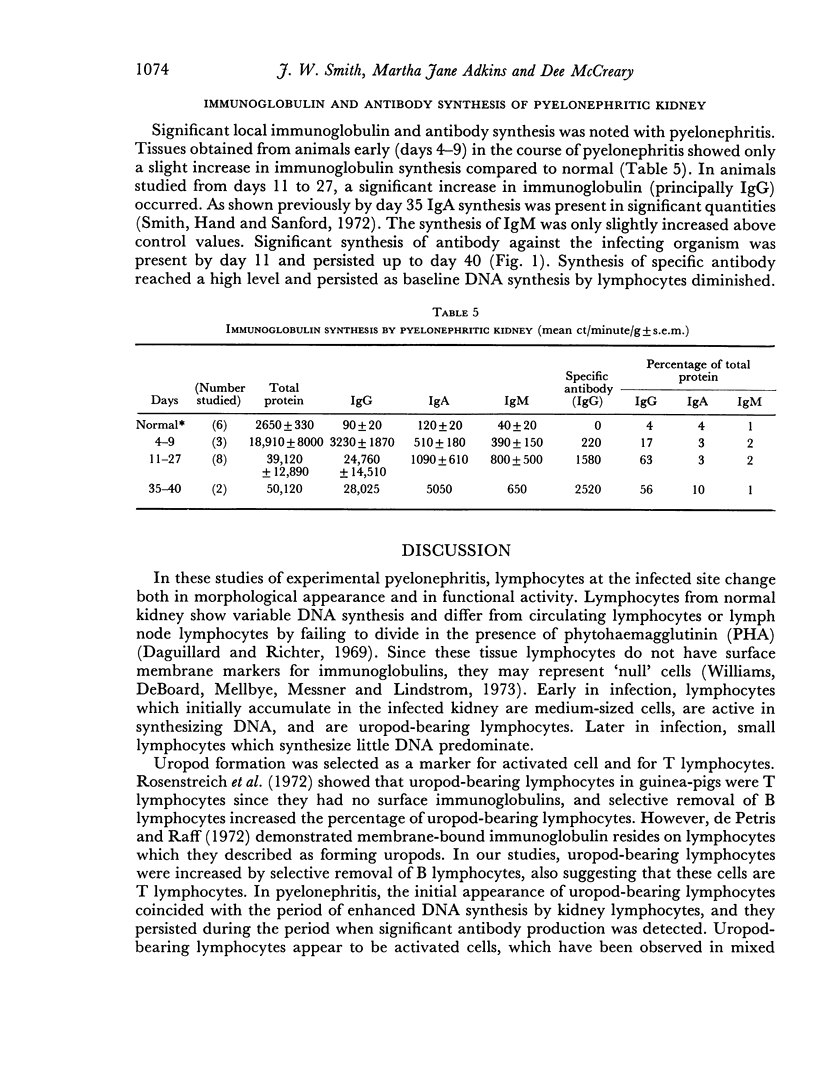


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld P. Uropod formation in phytohaemagglutinin (PHA) stimulated lymphocytes. Exp Cell Res. 1971 Jun;66(2):433–445. doi: 10.1016/0014-4827(71)90698-7. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Macrophages and delayed-type hypersensitivity. Semin Hematol. 1970 Apr;7(2):215–224. [PubMed] [Google Scholar]
- Coles G. A., Chick S., Hopkins M., Ling R., Radford N. J. The role of the T cell in experimental pyelonephritis. Clin Exp Immunol. 1974 Apr;16(4):629–636. [PMC free article] [PubMed] [Google Scholar]
- Daguillard F., Richter M. Cells involved in the immune response. XII. The differing responses of normal rabbit lymphoid cells to phytohemagglutinin, goat anti-rabbit immunoglobulin antiserum and allogeneic and xenogeneic lymphocytes. J Exp Med. 1969 Nov 1;130(5):1187–1208. doi: 10.1084/jem.130.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Hand W. L., Smith J. W., Miller T. E., Barnett J. A., Sanford J. P. Immunoglobulin synthesis in lower urinary tract infection. J Lab Clin Med. 1970 Jan;75(1):19–29. [PubMed] [Google Scholar]
- Janis M., Back F. H. Potentiation of in vitro lymphocyte reactivity. Nature. 1970 Jan 17;225(5229):238–239. doi: 10.1038/225238a0. [DOI] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. D., Smith J. W., Miller T. E., Barnett J. A., Sanford J. P. Local immune response in experimental pyelonephritis. J Clin Invest. 1969 Nov;47(11):2541–2550. doi: 10.1172/JCI105936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFARLAND W., HEILMAN D. H. LYMPHOCYTE FOOT APPENDAGE: ITS ROLE IN LYMPHOCYTE FUNCTION AND IN IMMUNOLOGICAL REACTIONS. Nature. 1965 Feb 27;205:887–888. doi: 10.1038/205887a0. [DOI] [PubMed] [Google Scholar]
- McFarland W. Microspikes on the lymphocyte uropod. Science. 1969 Feb 21;163(3869):818–820. doi: 10.1126/science.163.3869.818. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Simpson G., Ormrod D. J. Quantitation of immunoglobulin-bearing lymphocytes and the lymphocyte response to PHA in experimental pyelonephritis. Clin Exp Immunol. 1975 Sep;21(3):474–484. doi: 10.1002/aic.690210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Smith J. W., Sanford J. P. Antibody synthesis in kidney, spleen and lymph nodes in acute and healed focal pyelonephritis. Br J Exp Pathol. 1971 Dec;52(6):678–683. [PMC free article] [PubMed] [Google Scholar]
- Montgomerie J. Z., Kalmanson G. M., Guze L. B. Pyelonephritis: an attempt to demonstrate renal autoimmunity. N Z Med J. 1969 Oct;70(449):244–246. [PubMed] [Google Scholar]
- Newberry W. M., Sanford J. P. Defective cellular immunity in renal failure: depression of reactivity of lymphocytes to phytohemagglutinin by renal failure serum. J Clin Invest. 1971 Jun;50(6):1262–1271. doi: 10.1172/JCI106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Shevach E., Green I., Rosenthal A. S. The uropod-bearing lymphocyte of the guinea pig. Evidence for thymic origin. J Exp Med. 1972 May 1;135(5):1037–1048. doi: 10.1084/jem.135.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Hand W. L., Sanford J. P. Local synthesis of secretory IgA in experimental pyelonephritis. J Immunol. 1972 Apr;108(4):867–876. [PubMed] [Google Scholar]
- Smith J., Holmgren J., Ahlstedt S., Hanson L. A. Local antibody production in experimental pyelonephritis: amount, avidity, and immunoglobulin class. Infect Immun. 1974 Sep;10(3):411–415. doi: 10.1128/iai.10.3.411-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys H. D., Brody J. I. Altered lymphocyte reactivity to E. coli in chronic pyelonephritis. J Lab Clin Med. 1968 Jun;71(6):989–998. [PubMed] [Google Scholar]
- Swenson R. M., Kern M. THE SYNTHESIS AND SECRETION OF gamma-GLOBULINS BY LYMPH NODE CELLS, I. THE MICROSOMAL COMPARTMENTALIZATION OF gamma-GLOBULINS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):417–422. doi: 10.1073/pnas.57.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]