Abstract
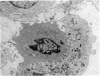
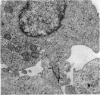
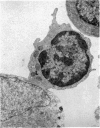
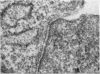
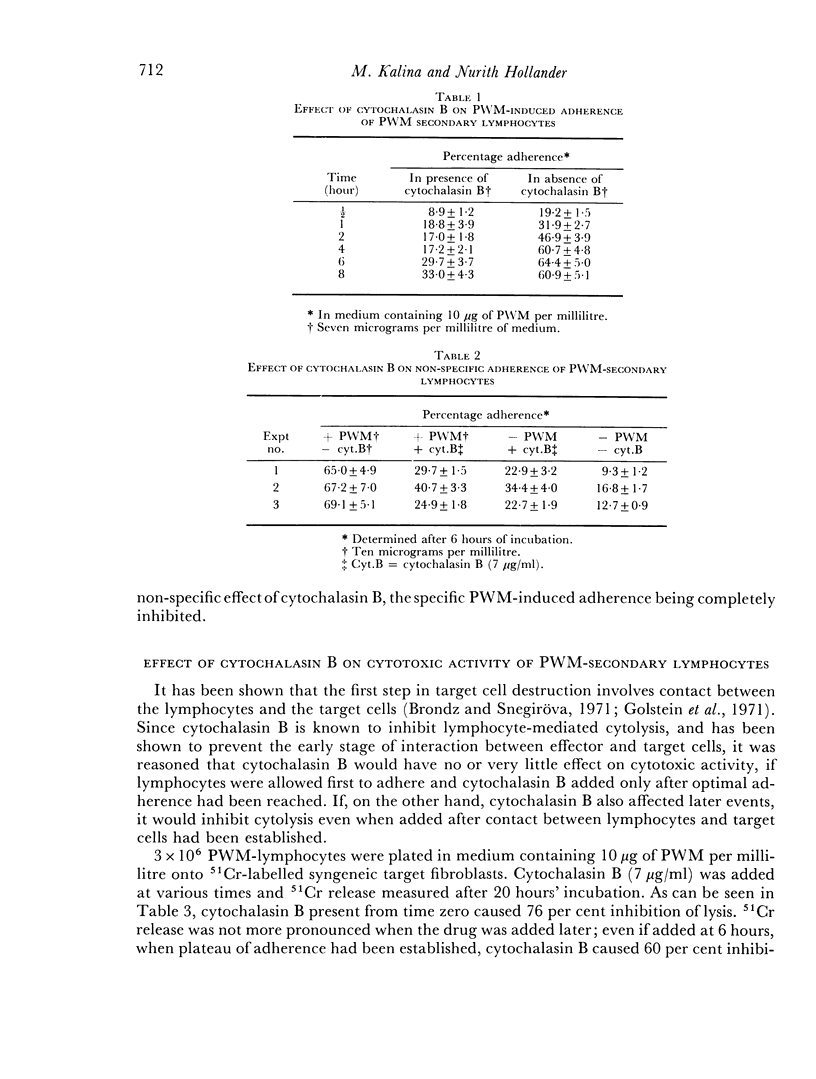
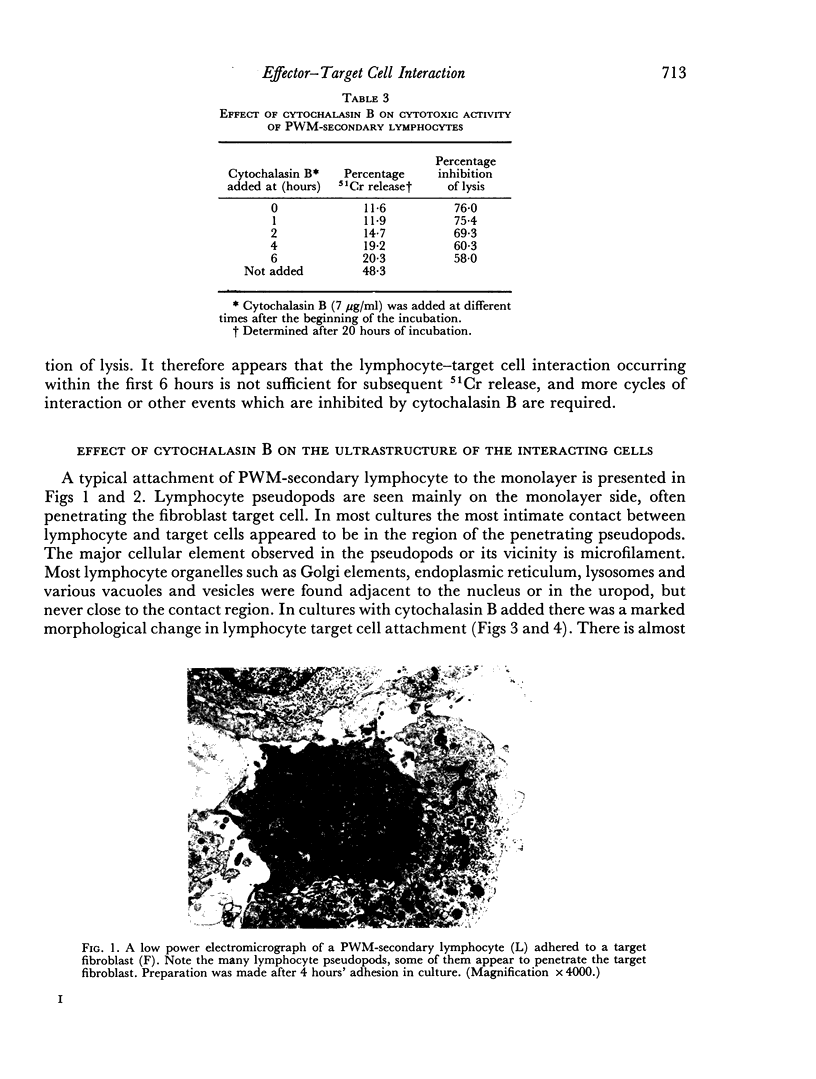
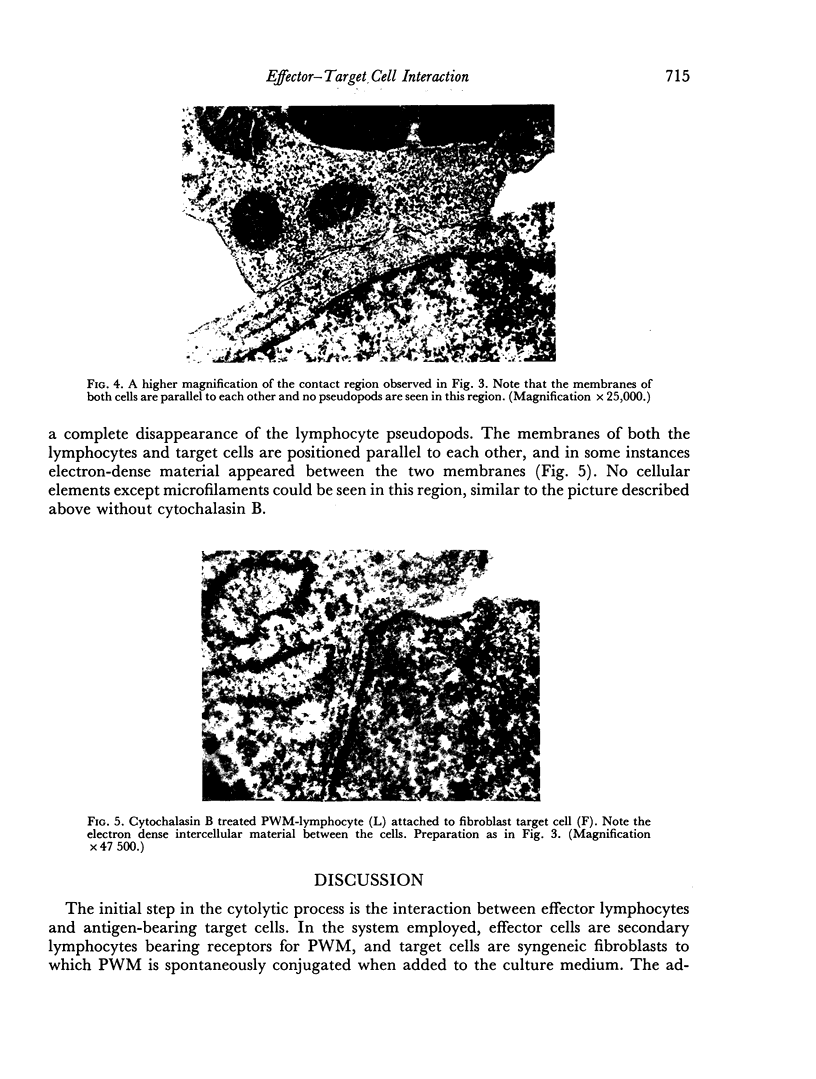
Interaction between pokeweed mitogen-stimulated secondary lymphocytes (PWM-lymphocytes) and target fibroblasts resulted in over 80 per cent adherence of the sensitized lymphocytes to the target cells. Adherence is by pseudopod penetration into target fibroblasts. The only lymphocyte cellular components found in the contact region were microfilaments. Cytochalasin B completely inhibited the specific adsorption of the PWM-secondary lymphocytes to the target cells. What adhesion did take place in the presence of cytochalasin B was found to be nonspecific. Ultrastructurally, the contact between lymphocyte and target cells was altered by the drug, when pseudopods were not observed. Possible effects of cytochalasin B on lymphocyte-mediated cytolysis, mainly by its effect on microfilament function, is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berke G., Ax W., Ginsburg H., Feldman M. Graft reaction in tissue culture. II. Quantification of the lytic action on mouse fibroblasts by rat lymphocytes sensitized on mouse embryo monolayers. Immunology. 1969 May;16(5):643–657. [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O. Microfilaments and cytoplasmic streaming: inhibition of streaming with cytochalasin. J Cell Sci. 1973 Jan;12(1):327–343. doi: 10.1242/jcs.12.1.327. [DOI] [PubMed] [Google Scholar]
- Brondz B. D., Snegiröva A. E. Interaction of immune lymphocytes with the mixtures of target cells possessing selected specificities of the H-2 immunizing allele. Immunology. 1971 Apr;20(4):457–468. [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- Diener E., Paetkau V. H. Antigen recognition: early surface-receptor phenomena induced by binding of a tritium-labeled antigen. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2364–2368. doi: 10.1073/pnas.69.9.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Hollander N., Feldman M. The development of hypersensitive lymphocytes in cell culture. J Exp Med. 1971 Oct 1;134(4):1062–1082. doi: 10.1084/jem.134.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Sachs L. Destruction of mouse and rat embryo cells in tissue culture by lymph node cells from unsensitized rats. J Cell Physiol. 1965 Oct;66(2):199–219. doi: 10.1002/jcp.1030660207. [DOI] [PubMed] [Google Scholar]
- Ginsburg H. The function of the delayed sensitivity reaction as revealed in the graft reaction culture. Adv Cancer Res. 1970;13:63–95. doi: 10.1016/s0065-230x(08)60164-5. [DOI] [PubMed] [Google Scholar]
- Golstein P., Erik M. D., Svedmyr A. J., Wigzell H. Cells mediating specific in vitro cytotoxicity. I. Detection of receptor-bearing lymphocytes. J Exp Med. 1971 Dec 1;134(6):1385–1402. doi: 10.1084/jem.134.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bubbers J. E. Antigen-T lymphocyte interactions: inhibition by cytochalasin B. J Immunol. 1973 Jul;111(1):85–90. [PubMed] [Google Scholar]
- Hollander N., Ginsburg H. Specific adherence of in vitro differentiated lymphocytes to target cells. J Exp Med. 1972 Dec 1;136(6):1344–1355. doi: 10.1084/jem.136.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. 3. The role of microfilaments and microtubules. J Immunol. 1973 Mar;110(3):771–780. [PubMed] [Google Scholar]
- Schofield J. G. Cytochalasin B and release of growth hormone. Nat New Biol. 1971 Sep 15;234(50):215–216. doi: 10.1038/newbio234215a0. [DOI] [PubMed] [Google Scholar]
- Sellin D., Wallach D. F., Fischer H. Intercellular communication in cell-mediated cytotoxicity. Fluorescein transfer between H-2 d target cells and H-2 b lymphocytes in vitro. Eur J Immunol. 1971 Dec;1(6):453–458. doi: 10.1002/eji.1830010609. [DOI] [PubMed] [Google Scholar]
- Thomas D des S., Lutzac M., Manavathu E. Cytochalasin selectively inhibits synthesis of a secretory protein, cellulase, in Achlya. Nature. 1974 May 10;249(453):140–142. doi: 10.1038/249140a0. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M., Waksman B. H., Malawista S. E. Cytochalasin B inhibits lymphotoxin production by antigen-stimulated lymphocytes. Science. 1972 Jun 9;176(4039):1147–1149. doi: 10.1126/science.176.4039.1147. [DOI] [PubMed] [Google Scholar]