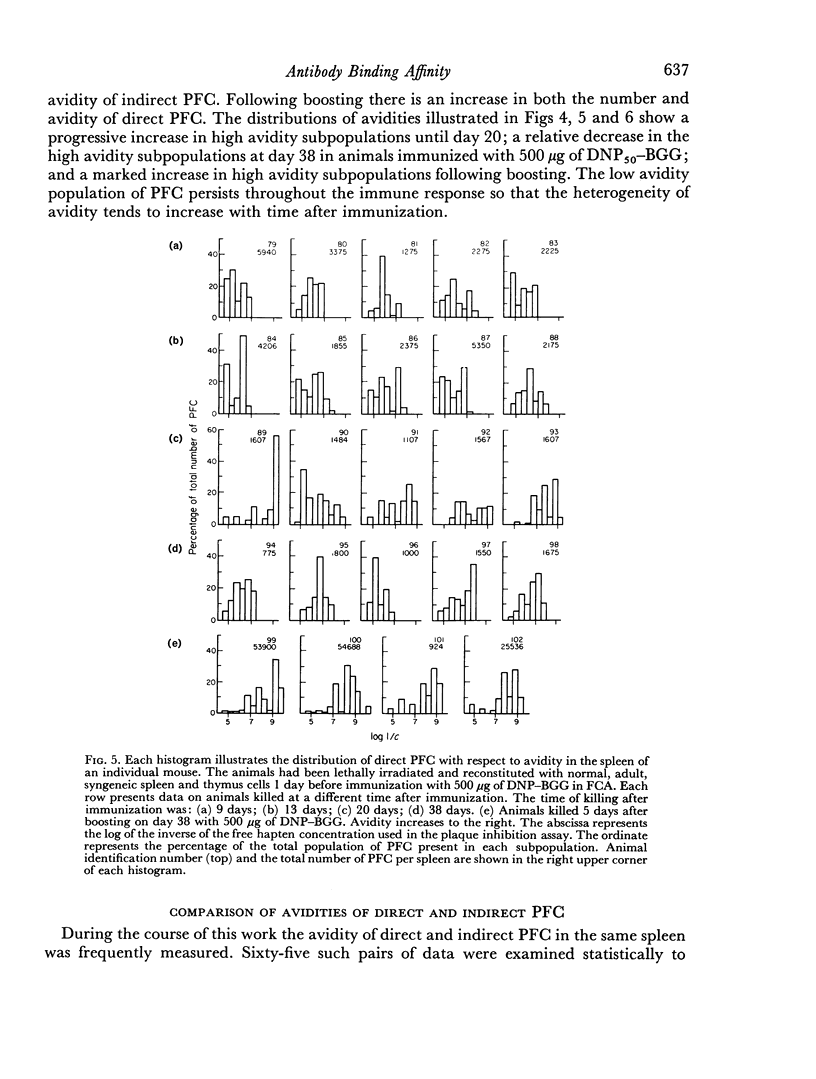
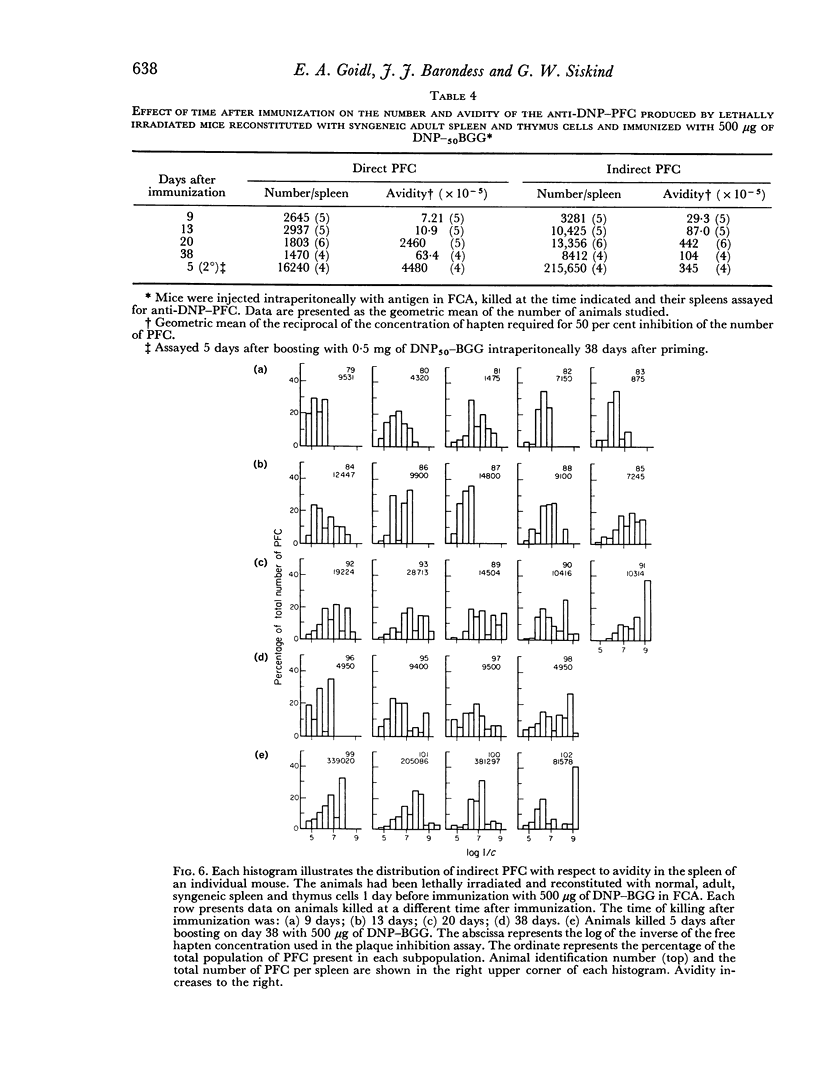
Abstract
The change in avidity of anti-hapten antibody with time after immunization was studied in mice at the level of the antibody-forming cell. A progressive increase in avidity was seen in both direct and indirect plaque-forming cells. Late (38 days) after immunization with a large dose of antigen there was a preferential loss of high avidity plaque-forming cells and the average avidity decreased. High avidity memory cells were still present since boosting resulted in the prompt appearance of very high avidity plaque-forming cells. There was a strong positive correlation between the avidity of the direct and the indirect plaque-forming cells present in the same spleen. A pattern of change in avidity and heterogeneity of avidity similar to that observed with intact animals was seen in lethally irradiated mice reconstituted with normal spleen and thymus cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. Studies on antibody affinity at the cellular level. Correlation between binding properties of secreted antibody and cellular receptor for antigen on immunological memory cells. J Exp Med. 1972 Feb 1;135(2):312–322. doi: 10.1084/jem.135.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin L., Merchant B., Inman J. Antibody-binding characteristics at the cellular level. I. Comparative maturation of hapten-specific IgM and IgG plaque-forming cell populations. J Immunol. 1973 Jan;110(1):241–251. [PubMed] [Google Scholar]
- Claflin L., Merchant B. Restricted maturation of antibody-binding characteristics for hapten-specific IgM-plaque-forming cells in mice. Cell Immunol. 1972 Sep;5(1):209–220. doi: 10.1016/0008-8749(72)90097-4. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. V. Cellular correlates of the "maturation" of the immune response. J Exp Med. 1972 Mar 1;135(3):660–674. doi: 10.1084/jem.135.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Goldstein B. On the mechanism of hemolytic plaque inhibition. Immunochemistry. 1974 Oct;11(10):661–665. doi: 10.1016/0019-2791(74)90223-7. [DOI] [PubMed] [Google Scholar]
- Doria G., Schiaffini G., Garavini M., Mancini C. The rise and fall of antibody avidity at the level of single immunocytes. J Immunol. 1972 Dec;109(6):1245–1253. [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Paul W. E., Siskind G. W., Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968 Feb;100(2):371–375. [PubMed] [Google Scholar]
- Huchet R., Feldmann M. Studies on antibody affinity in mice. Eur J Immunol. 1973 Jan;3(1):49–55. doi: 10.1002/eji.1830030111. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Siskind G. W. Studies on the control of antibody synthesis. VI. Effect of antigen dose and time after immunization on antibody affinity and heterogeneity in the mouse. Clin Exp Immunol. 1974 Jun;17(2):329–338. [PMC free article] [PubMed] [Google Scholar]
- Lamelin J. P., Paul W. E. Rate of increase of antibody affinity in different rat strains. Int Arch Allergy Appl Immunol. 1971;40(3):351–360. doi: 10.1159/000230418. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Segre D. Determination of relative affinity and heterogeneity of mouse anti-DNP antibodies by a plaque-inhibition technique. J Immunol. 1972 Jul;109(1):74–83. [PubMed] [Google Scholar]
- Paul W. E., Yoshida T., Benacerraf B. Genetic control of the specificity of anti-DNP antibodies. II. Differences in the specificity of anti-DNP antibody produced by several inbred strains of mice. J Immunol. 1970 Aug;105(2):314–321. [PubMed] [Google Scholar]
- Roszman T. L. Analysis of the temporal changes in the avidity of primary IgM and IgG antibody at the cellular level. Cell Immunol. 1974 Mar 30;11(1-3):205–211. doi: 10.1016/0008-8749(74)90020-3. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Siskind G. W., Dunn P., Walker J. G. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J Exp Med. 1968 Jan 1;127(1):55–66. doi: 10.1084/jem.127.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Kim Y. T., Quagliata F., Siskind G. W. Studies on the control of antibody synthesis. 3. Changes in heterogeneity of antibody affinity during the course of the immune response. Immunology. 1973 Mar;24(3):477–492. [PMC free article] [PubMed] [Google Scholar]
- Werblin T. P., Siskind G. W. Effect of tolerance and immunity on antibody affinity. Transplant Rev. 1972;8:104–136. doi: 10.1111/j.1600-065x.1972.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Cinader B. A cell population study of IgM affinity maturation. Cell Immunol. 1973 Aug;8(2):189–197. doi: 10.1016/0008-8749(73)90109-3. [DOI] [PubMed] [Google Scholar]
- Wu C. Y., Cinader B. Dose- and time-dependent changes in the binding capacity of IgM antibody. Eur J Immunol. 1972 Oct;2(5):398–405. doi: 10.1002/eji.1830020503. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yamada A., Hollander V. P. 2,4-dinitrophenyl-hapten specific hemolytic plaque-in-gel formation by mouse myeloma (MOPC-315) cells. J Immunol. 1970 Jan;104(1):251–255. [PubMed] [Google Scholar]


