Abstract
Rac3, a neuronal GTP-binding protein of the Rho family, induces neuritogenesis in primary neurons. Using yeast two-hybrid analysis, we show that Neurabin I, the neuronal F-actin binding protein, is a direct Rac3-interacting molecule. Biochemical and light microscopy studies indicate that Neurabin I copartitions and colocalizes with Rac3 at the growth cones of neurites, inducing Neurabin I association to the cytoskeleton. Moreover, Neurabin I antisense oligonucleotides abolish Rac3-induced neuritogenesis, which in turn is rescued by exogenous Neurabin I but not by Neurabin I mutant lacking the Rac3-binding domain. These results show that Neurabin I mediates Rac3-induced neuritogenesis, possibly by anchoring Rac3 to growth cone F-actin.
INTRODUCTION
During early differentiation neurons extend neurites, cylindrical extensions without clear morphological and molecular features of an axon or a dendrite. One of the neurites then becomes the neuronal axon, which navigates for long distances before reaching its targets, guided by attractive and repulsive, long- and short-range axon guidance signals present in the extracellular environment (Tessier-Lavigne and Goodman, 1996; Orioli and Klein, 1997; Yu and Bargmann, 2001). Guidance molecules bind to membrane receptor proteins exposed by the distal end of extending neurites, known as growth cones, and activate intracellular signaling cascades that cause growth cone reorientation (Song and Poo, 2001). The movement of the growth cone occurs through the extension and retraction of filopodia and lamellipodia that are mainly composed of actin filaments (F-actin) and that move by rearranging actin cytoskeleton (da Silva and Dotti, 2002). F-actin is assembled at the leading edge of the growth cone, transported into the center of the cone, and then disassembled. This retrograde flow of F-actin seems to be crucial for growth cone extension, turning, and retraction (Tanaka and Sabry, 1995).
The molecular mechanisms linking membrane receptors to actin dynamics in neurons are still poorly understood. Several studies have shown a role for Rho GTP-binding molecules in regulating actin cytoskeleton in a variety of cell types, including neuronal cells (Luo, 2000; Dickson, 2001; Giniger, 2002). Among the Rho proteins, the function of RhoA, Rac1, and Cdc42 has been extensively investigated. Microinjection experiments in fibroblasts have shown a role for RhoA in regulating actin-stress-fibers and focal adhesions, Rac1 in regulating membrane ruffling and lamellipodia, and Cdc42 in regulating filopodia (Hall, 1998).
Rho proteins belong to the Ras superfamily, and similarly to Ras, they cycle between an active GTP-bound and an inactive GDP-bound state. The ratio between the active and inactive states seems to be regulated by three classes of protein regulators: guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP to GTP; GTPase-activating proteins (GAPs), which stimulate the intrinsic Rho GTPase activity; and guanine nucleotide dissociation inhibitors (GDIs), which bind to Rho proteins inhibiting the nucleotide exchange (Van Aelst and D'Souza-Schorey, 1997; Symons and Settleman, 2000; Schmidt and Hall, 2002). Rho GTP-bound molecules activate effector proteins that further transduce the signaling cascade and operate on several cellular events, such as membrane trafficking, transcriptional regulation, cell growth, and development (Aspenstrom, 1999). During neuritogenesis, the use of constitutive active or dominant negative mutants has revealed an involvement of Rho proteins in axon and dendrite formation (Luo et al., 1996). Several axon guidance molecules have been shown to signal through the Rho proteins (Wahl et al., 2000; Driessens et al., 2001; Shamah et al., 2001), indicating that these molecules might play a role in actin cytoskeleton rearrangements during neurite extension. The mechanisms by which Rho proteins mediate growth cone extension, turning, and retraction are not completely understood.
Rac3, also known as Rac1B, is a member of the Rho family and is preferentially expressed in neuronal cells (Haataja et al., 1997; Malosio et al., 1997). During chicken development, Rac3 shows the highest expression at the time of neuritogenesis and synaptogenesis; Rac3 overexpression induces neurite extension and neurite branching in chicken retinal ganglion cells (Albertinazzi et al., 1998). However, the molecular mechanism by which Rac3 induces neuritogenesis is unknown. To address this issue, we have searched for novel Rac3 interacting proteins. One of the proteins we identified is Neurabin I. Neurabin I was originally isolated as a neuronal protein that binds along the sides of F-actin and that shows F-actin cross-linking activity (Nakanishi et al., 1997). It localizes at the lamellipodia of growth cone in developing neurons, at the synapse of differentiated neurons (Nakanishi et al., 1997), and in the spines of neuronal dendrites (Muly et al., 2004). Expression of a Neurabin I deletion mutant was found to stimulate F-actin polymerization and the motility of dendritic spines, leading to increase spine morphogenesis and synapse formation (Zito et al., 2004). In the present article, we investigate the presence of Rac3 and Neurabin I in the growth cone of extending neurites, the nature of interaction between Rac3 and Neurabin I, and their role in neuritogenesis.
MATERIALS AND METHODS
Plasmids
The hemagglutinin (HA) epitope tag was inserted at the 5′ of rat Neurabin I cDNA and cloned into pCDNA3, by standard PCR procedure. Rac3ΔC-ter and Neurabin I mutants were generated by PCR-driven mutagenesis and sequenced.
Yeast Two-Hybrid Screen and Mating
Rac3 cDNA was cloned into pAS2 (pAS1-CYH2) vector to generate the bait construct expressing Rac3-GAL4 DNA binding domain (BD) fusion protein. In total, 3.2 × 106 Y190 yeast cells expressing the bait were transformed with E11 mouse embryo cDNA library (Clontech, Mountain View, CA) fused to the sequence of the GAL4-activation domain (AD) (pACT2 vector) and selected as described previously (Bruckner et al., 1999) in the presence of 37 mM 3-amino triazole (Sigma-Aldrich, St. Louis, MO). Each positive clone was selected with 10 mM cycloheximide-containing medium to lose the bait. These cured strains were mated with an equivalent number of Y187 yeast cells expressing the GAL4 BD alone or fused to Rac3- or Rac3N17 proteins. Matings were assayed for β-galactosidase activity. Isolated plasmids were sequenced, and cDNA fragments were identified by BLAST analysis against the GenBank.
Cell Culture and Transfection
Neurons were isolated from the hippocampus of E18 fetal rat brains and prepared as described previously (Goslin and Banker, 1991). SK-N-BE cells were grown and induced to differentiate by retinoic acid (RA) as described previously (Peverali et al., 1996). SK-N-BE cells were transfected by 4-h incubation with Lipofectamine 2000 in Opti-MEM (Invitrogen, Carlsbad, CA), cultured in standard medium and analyzed 28-48 h later. For oligonucleotide experiments, neuroblastoma (NB) cells were cultured for three sequential incubations of 12 h in 2 μM sense or antisense Neurabin I (-17; +3) phosphorothioate oligonucleotides (PONs) (MWG-Biotech, Ebersberg, Germany) containing medium. Human embryonic kidney (HEK)293 cells were cultured and transfected by calcium phosphate method as described previously (Gabellini et al., 2003).
Growth Cone Isolation
Growth cone particles were prepared from E18 rat brains (Pfenninger et al., 1983; Lohse et al., 1996). Briefly, fetal brains were homogenized in 8 volumes of 0.32 M sucrose containing 1 mM MgCl2, 1 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES) buffer, pH7.3, and protease inhibitors. A low-speed (1860 × g for 16 min; rotor SS34) supernatant was loaded onto a discontinuous sucrose density gradient with steps of 0.83 and 2.66 M sucrose containing MgCl2 and TES. The gradient was spun to equilibrium at 242,000 × g for 45 min in a vertical rotor (Vti50; Beckman Coulter, Fullerton, CA). The growth cone particle fraction at the 0.32/0.83 M sucrose interfaces was collected, diluted with 4 volumes of 0.32 M sucrose containing MgCl2 and TES, and centrifuged for 30 min at 39,800 × g (rotor Ti70). This pellet was then used in subsequent experiments.
Immunoprecipitation, Western Blotting, and Antibodies
Cells were lysed in SDS-sample buffer (Laemmli buffer). For cytosolic and cytoskeletal proteins, SK-N-BE cells or pellet derived from the purification of neuronal growth cones were lysed in 0.3 ml of lysis buffer [50 mM Tris-HCl, pH 7.5, 90 mM NaCl, 0.5 mM EGTA, 1% Triton, 0.1 mM Na3VO4, 50 mM NaF, 20 mM p-nitrophenyl phosphate, 25 mM Na2β-glycerophosphate, and 0.2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride] supplemented with a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and centrifuged for 20 min at 13,000 rpm in Centrifuge (Eppendorf AG, Hamburg, Germany). Supernatants (S fraction) were harvested, and 1× SDS-sample buffer was added. Pellets (P fraction) were also resuspended in 1× SDS-sample buffer. For immunoprecipitation experiments, SK-N-BE and HEK293 cells were lysed in 230 and 280 mM sodium-containing lysis buffer, respectively. Immunoprecipitation lysates were incubated overnight at 4°C with 30 μl of agarose-conjugated-anti-FLAG antibodies (Sigma-Aldrich, St. Louis, MO) or with anti-Rac3 (sc-16698; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or with goat IgG antibodies coupled to protein A-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Cell extracts, cytosolic or cytoskeletal protein extracts, or immunoprecipitated proteins were fractionated onto SDS-PAGE and immunoblotted (Henkemeyer et al., 1996) with anti-Neurabin I (Sigma-Aldrich); anti-α-tubulin (Sigma-Aldrich); anti-β-actin (Sigma-Aldrich); anti-FLAG, anti-Rac3, and anti-HA (Roche Diagnostics); or anti-integrin β1 (BD Transduction Laboratories, Lexington, KY) antibodies.
Immunostaining and Confocal Microscopy
Hippocampal neurons or SK-N-BE cells were grown on poly-l-lysine-coated glass coverslips (Orioli et al., 1996), fixed 15 min with 3-4% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized 5 min with 0.2% Triton X-100 in PBS, and assayed with TRITC-phalloidin (Sigma-Aldrich), with tetramethylrhodamine B isothiocyanate (TRITC)-conjugated anti-HA (Roche Diagnostics) or fluorescein isothiocyanate (FITC)-conjugated FLAG (Sigma-Aldrich) antibodies and by indirect immunofluorescence with Rac3 or Neurabin I antibodies. Fluorescent secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). After the desired treatment, cells were visualized by Leica TCSSP2 confocal microscopy or by an Olympus IX71 inverted fluorescence microscope equipped with XF202, XF204, and XF208 filters (www.Omegafilters.com) and with Photometrics CoolSnap ES (Roper Scientific, Trenton, NJ) cooled charge-coupled device camera. MetaMorph Imaging version 6.2 (Molecular Devices, Sunnyvale, CA) was used for data acquisition.
RESULTS
Rac3 Colocalizes with F-Actin in the Neurites of Neuronal Cells
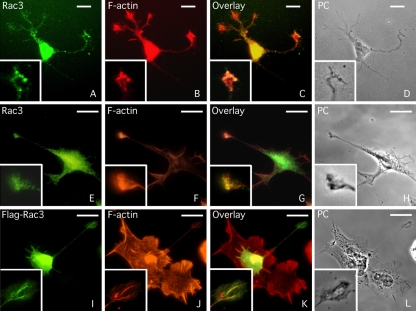
Overexpression of Rac3 in retinal ganglion cells induces the formation and increases the branching of new neurites (Albertinazzi et al., 1998). To gain further knowledge of the Rac3 protein and to better understand the signaling cascade of Rac3-induced neuritogenesis, the cellular distribution of Rac3 was observed in primary cell cultures of rat hippocampal neurons, incubated with FITC-conjugated anti-Rac3 antibodies. Confocal microscopy analysis revealed the presence of Rac3 throughout the cell body and also in neurites, without any apparent polarized compartmentalization. In growth cones, the signal seemed stronger and limited in the F-actin-enriched area of these structures (Figure 1, A-D), consistent with previous studies showing the colocalization of Rac3 with F-actin in the terminal axons of Purkinje cells (Bolis et al., 2003). Notably, Rac3 staining was undetectable in some neurites under these experimental conditions. Next, Rac3 staining was investigated in human NB SK-N-BE cells. NB is a neuroectodermal tumor originating from neural crests, and similarly to primary neurons, NB-derived cells can differentiate in vitro by extending long and branched neurites that are capable of establishing functional synaptic contacts after treatment with several agents, including RA (Abemayor and Sidell, 1989; Peverali et al., 1996). In RA-differentiated NB cells, Rac3 was detectable in the cell body, in the neurites and in some growth cones, where it also colocalized with F-actin (Figure 1, E-H), confirming the cellular distribution observed in primary neurons. Furthermore, in NB cells transfected with a Flagged-Rac3 expression vector and analyzed by immunofluorescence, anti-FLAG antibodies confirmed the presence of Rac3 at the tip of neurites, where it colocalized with F-actin (Figure 1, I-L). Interestingly, FlagRac3-positive cells extended long and branched neurites even more rapidly than RA-differentiated NB cells (Figure 1; see below), confirming that Rac3 plays a key role in neurite elongation.
Figure 1.
Rac3 colocalizes with F-actin in the neurites and in several growth cones of neuronal cells. (A-D) Immunofluorescence staining of embryonic rat hippocampal neurons with TRITC-conjugated phalloidin to reveal the F-actin distribution and with goat anti-Rac3 antibodies followed by FITC-conjugated anti-goat antibodies. (E-H) Human neuroblastoma SK-N-BE cells were differentiated by three days of RA treatment and stained as described above. (I-L) SK-N-BE cells were transfected with FlagRac3 expression vector that stimulates the formation of long and branched neurites. NB cells were stained with TRITC-conjugated phalloidin and immunostained with FITC-conjugated anti-FLAG antibodies. The same field is shown in phase contrast (PC). In each photogram, higher magnification of a growth cone is shown. Bar, 10 μm.
Neurabin I Is a Novel Rac3-interacting Molecule
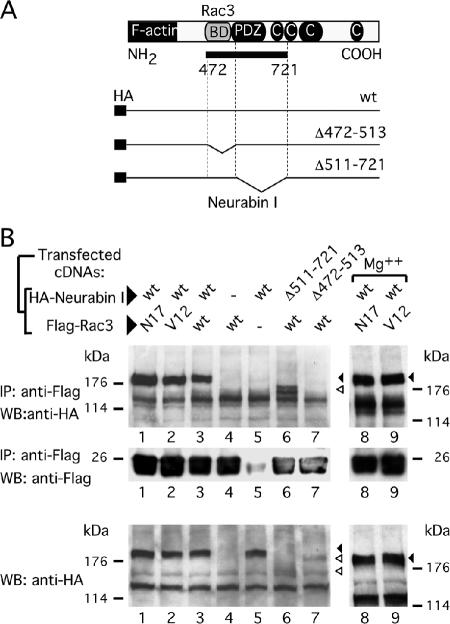
To unveil the molecular mechanisms by which Rac3 participates in neuritogenesis, we searched for neuronal Rac3 interacting proteins. To this purpose, an embryonic mouse cDNA library was screened by yeast two-hybrid system. Several positive clones were isolated showing histidine autotrophy and β-galactosidase activity. Clones #26, #119, and #136 presented an identical DNA fragment of 800 base pairs that shared 92% homology with the rat Neurabin I sequence, whereas the translated sequence showed 100% identity with the Neurabin I protein (Nakanishi et al., 1997). Neurabin I is a neuronal protein that contains a F-actin binding domain at the NH2-terminal region; a PSD-95, DlgA, ZO-1-like (PDZ) domain in the central region; and four separated domains at the COOH-terminal region of the protein that were predicted to mediate coiled-coil structures. The isolated cDNA fragment, named Neurabin I (472-721), encodes from amino acid 472 to 721 of Neurabin I and contains the PDZ domain (a.a. 505-595) flanked by two stretches of 33 and 126 amino acids (Figure 3A). Yeast mating experiments were carried out, to further investigate the Rac3/Neurabin I interaction and the role of the guanine nucleotides bound to Rac3. Initially, the Y190 yeast strain was transformed with the vector expressing Neurabin I (472-721) fused to GAL4-activation domain; this strain was then mated to Y187 yeast strains expressing the GAL4-DNA binding domain fused to either Rac3 or the dominant negative mutant Rac3N17. The constitutive active mutant Rac3V12 was found to be toxic to the cells and could not be assayed. The interaction between Neurabin I (472-721) and Rac3 or Rac3N17 allowed reconstitution of GAL4 transcription factor that transactivated the β-galactosidase reporter gene. As such, positive colonies stained blue in the β-galactosidase assay. In the two matings, comparable number of blue colonies was observed, suggesting that Neurabin I (472-721) may equally bind Rac3 and Rac3N17, without showing any preference for the GTP or GDP loaded nucleotides (Table 1). Yeast two-hybrid screening allowed to isolate other cDNAs whose products interacted with Rac3. Clone #11 is a partial cDNA coding for the CdGAP protein (residues 417-502). CdGAP is a GAP regulator of Rac1 and Cdc42 (Lamarche-Vane and Hall, 1998). Furthermore, clones #6 and #23 encoded for the entire RhoGDI-1 protein that interacted with the GDP-bound Rac1 (Driessens et al., 2001). Because Rac3 differs from Rac1 by only 12 amino acids, it is therefore reasonable and expected that known Rac1-interacting proteins were also isolated in the yeast two-hybrid system. When CdGAP-expressing yeast cells were mated to Rac3- or Rac3N17-expressing yeast cells, more blue colonies were observed in the CdGAP-Rac3 mating compared with the CdGAP-Rac3N17 (Table 1). These data suggest that CdGAP may interact with the active form of Rac3, as previously shown for Cdc42 and Rac1 (Lamarche-Vane and Hall, 1998). The identification of the Rac1 interacting molecules confirmed the strength of the screening and the validity of the matings, without however proving that this also occurs in vivo in mammalian cells.
Figure 3.
The Rac3-binding region is located close to the PDZ domain of Neurabin I. (A) Schematic representation of Neurabin I protein showing the F-actin binding, the PDZ, and the coiled-coil domains. The Rac3-binding domain (Rac3 BD) is shown in gray. A solid bar indicates the 250-amino acid-long fragment of Neurabin I (a.a. 472-721) isolated by yeast two-hybrid system. The full-length wild-type and the deletion mutants of HA-tagged Neurabin I cDNA are also depicted. (B) HEK293 cells were cotransfected with the indicated HANeurabin I and FlagRac3 expression vectors. Anti-FLAG-antibodies were used to coimmunoprecipitate the FlagRac3-associated recombinant proteins. Anti-HA immunoblots (top) reveal that HANeurabin I and HANeurabin I Δ511-721 associate with FlagRac3, whereas HANeurabin I Δ472-513 does not. Wild-type HANeurabin I associates with Rac3N17 and Rac3V12 with or without addition of Mg2+. Anti-FLAG immunoblots (middle) show the recovery of Flagged-proteins. Anti-HA immunoblot (bottom) is the control to show the levels of the transfected wild-type and mutated HANeurabin I proteins. Black and empty arrowheads indicate wild type HANeurabin I and HANeurabin I mutants, respectively.
Table 1.
Rac3 and Neurabin I interaction in yeast mating experiments
| Yeast Y190a prey | Yeast Y187b bait | 3-ATc (37 mM) | Leu/His/Trpd autotrophy | β-Gale activity |
|---|---|---|---|---|
| pAS2-Rac3 | – | – | – | |
| pACT2 | – | – | – | |
| pACT2 | pAS2-Rac3 | + | – | – |
| pACT2-Neurabin I472-721 | pAS2 | + | – | – |
| pACT2-Neurabin I472-721 | pAS2-Rac3 | + | +++ | +++ |
| pACT2-Neurabin I472-721 | pAS2-Rac3N17 | + | +++ | +++ |
| pACT2-CdGAP | pAS2 | + | – | – |
| pACT2-CdGAP | pAS2-Rac3 | + | ++ | ++ |
| pACT2-CdGAP | pAS2-Rac3N17 | + | + | + |
| pSE 1111f | – | – | – | |
| pSE 1112f | – | – | – | |
| pSE 1111 | pSE 1112 | + | ++ | ++ |
The cDNAs of the isolated prey, in frame with the GAL4-AD sequence, were expressed in the Y190 yeast strain
The cDNAs of the indicated baits were cloned in frame with the GAL4-BD sequence and expressed in the Y187 yeast strain
Molar concentration of 3-amino triazole
Colonies grown in selective medium are indicated by + (<50 colonies), ++ (>100), or +++ (>1000)
Number of β-galactosidase positive (blue) colonies
SNF4 fused to the GAL4-AD (pSE1111) and SNF1 fused to the GAL4-BD (pSE1112) are positive controls of protein–protein interaction
Neurabin I Coimmunoprecipitates with Rac3
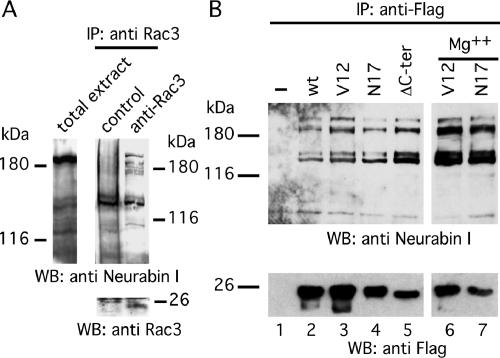
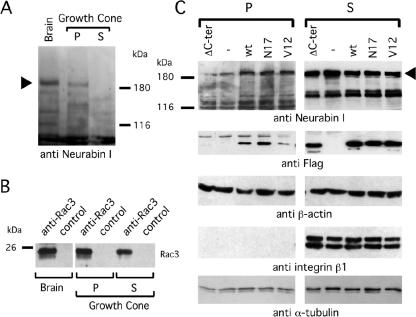
To confirm that Rac3/Neurabin I interaction also occurs in vivo during neuritogenesis, an immunoprecipitation experiment was performed in human cells. First, however, the expression of Neurabin I in NB total cell lysate was verified by immunoblot. This revealed a major band, slightly higher than 180 kDa, and two weak protein bands of ∼140 kDa (Figure 2A). This is in agreement with the molecular weight of Neurabin I (Nakanishi et al., 1997). The 140-kDa doublet was also previously observed in total brain lysate and described as possible partial degradation product (Nakanishi et al., 1997). Having confirmed the existence of Neurabin I, anti-Rac3 antibodies were used to immunoprecipitate the endogenous Rac3 and its associated proteins in RA-differentiated NB cells. Anti-Neurabin I immunoblot revealed the presence of Neurabin I among the Rac3-associated proteins (Figure 2A), further strengthening the view that these molecules are partners of the same complex. Two major bands of ∼180 kDa of Neurabin I (Figure 2A) and additional minor bands were observed that might correspond to different posttranslational modifications or partial proteolysis of Neurabin I as discussed previously (Nakanishi et al., 1997).
Figure 2.
Rac3 forms a complex with Neurabin I. (A) RA-differentiated SK-N-BE cells were lysed and immunoprecipitated with control IgG or anti-Rac3 antibodies. The complexes were fractionated onto SDS-PAGE and immunoblotted with anti-Neurabin I (top) or anti-Rac3 (bottom) antibodies. Anti-Neurabin I immunoblot of SK-N-BE total lysate is also shown (left). The relative molecular mass of a standard prestained molecular weight marker is shown next to the immunoblots. (B) SK-N-BE cells were transfected with vectors encoding for FlagRac3 or the indicated FlagRac3-mutants and lysed. The FLAG-tagged proteins were immunoprecipitated by anti-FLAG antibodies. The levels of the endogenous 180- and 140-kDa Neurabin I proteins associated to the FLAG-recombinant proteins are shown by anti-Neurabin I immunoblot (top). To equilibrate the loading of the GTP/GDP nucleotides on Rac3 proteins (see text), an aliquot of cells was lysed in the presence of 5 mM Mg2+ and processed as described above. The recovery of the Flagged-Rac3 proteins is shown by anti-FLAG immunoblot (bottom).
To understand the role of guanine nucleotides on Rac3/Neurabin I association, NB cells were transfected with the expression vector encoding for FlagRac3, FlagRac3V12 or FlagRac3N17. Anti-FLAG immunoprecipitations were fractionated on SDS-PAGE. Anti-Neurabin I immunoblot showed the association of Neurabin I to both wild-type and mutated forms of Rac3, indicating that Neurabin I interacts with active and inactive Rac3 (Figure 2B). To further investigate this, a parallel coimmunoprecipitation was carried out in the presence of 5 mM magnesium (Figure 2B), which guarantees the loading of the GTP or GDP nucleotides on the Rac3 mutants during the immunoprecipitation experiment (Meller et al., 2002). The results indicate that Neurabin I/Rac3 interaction is independent on the guanine nucleotides and on the active/inactive state of Rac3, consistent with the yeast mating results (Table 1). Last, we investigated whether Rac3/Neurabin I interaction requires Rac3-membrane binding. Because Rac-membrane binding requires the attachment of the 20-carbon geranylgeranyl moiety to the C-terminal cysteine residue (Cys 189), followed by the proteolysis of the last three C-terminal amino acids (190-192) (Seabra, 1998; Del Pozo et al., 2002), a Rac3ΔC-ter mutant, lacking the six C-terminal amino acids (a.a. 187-192), was generated. When assayed as above, the endogenous Neurabin I protein still coprecipitated with FlagRac3ΔC-ter (Figure 2B). The amount of Neurabin I coprecititated with FlagRac3ΔC-ter was even higher compared with that coprecipitated with the other FlagRac3 proteins, indicating the anchorage of Rac3 to the membrane is not required for this interaction (Figure 2B).
The Rac3-interacting Domain Is Adjacent to Neurabin I PDZ Domain
To map the Rac3-binding region on Neurabin I, PCR-driven mutagenesis was conducted on the full-length HA-tagged Neurabin I sequence (Figure 3; see Materials and Methods). To cover the entire protein fragment isolated with the yeast two-hybrid assay, two deletion mutants were generated that removed the 472-513 and 511-721 protein regions. The mutated and wild-type sequences of Neurabin I were transfected in cells together with FlagRac3, or FlagRac3N17 or FlagRac3V12. First, the levels of the recombinant proteins were analyzed by anti-Neurabin I immunoblot on total cell extracts. The levels of the mutant proteins were lower than the amount of the wild-type HANeurabin I. In particular HANeurabin I Δ511-721 showed a barely detectable band that comigrated just above a nonspecific band (Figure 3B, bottom, lane 6). In anti-FLAG immunoprecipitation assay, HANeurabin I Δ511-721 and wild-type Neurabin I coprecipitated with FlagRac3 (Figure 3B, top, lanes 3 and 6). Similarly, a PDZ deletion mutant (a.a. 513-595) coprecipitated with FlagRac3 (our unpublished data). In contrast, FlagRac3 failed to coprecipitate HANeurabin I Δ472-513 that lacked the 33 amino acids located at the N terminus of the PDZ domain and the first eight amino acids of the PDZ domain FlagRac3 (Figure 3B, top, lane 7). As a control, anti-FLAG immunoblot confirmed that FlagRac3 was present in the immunoprecipitated fraction (Figure 3B, middle, lane 7). These data demonstrate that the protein region in Neurabin I involved in Rac3 binding is located close to the PDZ domain, between amino acids 472 and 513. The PDZ domain seemed to be excluded from the Rac3 interaction, consistent with the observation that the majority of PDZ domains binds to the C-terminal tail of partner proteins (Saras and Heldin, 1996) and to the fact that Rac3ΔC-ter, lacking its C-terminal tail, still interacts with Neurabin I (Figure 2B, lane 5).
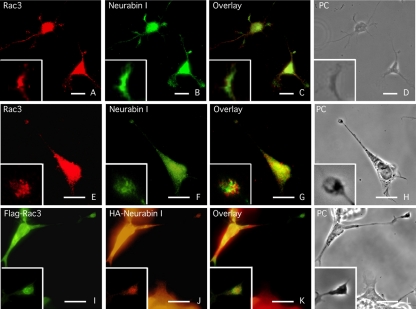
Neurabin I and Rac3 Colocalize at the Growth Cone of Extending Neurites
The cellular distributions of Rac3 and Neurabin I were next analyzed in primary cultures of rat hippocampal neurons and in RA-differentiated NB cells (Figure 4, A-H). In both cell types, Neurabin I was observed along the neurites and in several growth cones. The signal obtained by anti-Rac3 antibodies was weaker compared with Neurabin I. Nevertheless, the two proteins clearly colocalized in growth cones. Similar results were obtained when NB cells were cotransfected with the expression vectors encoding for FlagRac3 and HANeurabin I. These cells showed colocalization of both recombinant proteins at the tip of extending neurites (Figure 4, I-L).
Figure 4.
Rac3 and Neurabin I colocalize in the growth cone of extending neurites. Immunofluorescence staining of Rac3 and Neurabin I proteins in primary culture of embryonic rat hippocampal neurons (A-D) and in RA-differentiated SK-N-BE cells (E-H). Cells were immunostained with goat anti-Rac3 and rabbit anti-Neurabin I antibodies. Phase contrast (PC) image of the same field is shown. Overlay and detail photograms show Rac3 and Neurabin I colocalization at the tip of neurites. (I-L) Same as above stained with FITC-anti-FLAG and TRITC-anti-HA antibodies of FlagRac3 and HANeurabin I expressing SK-N-BE cells. Bar, 10 μm.
To confirm the presence of Neurabin I and Rac3 at the tip of extending neurites, neuronal growth cones were isolated from embryonic rat brains by subcellular fractionation (see Materials and Methods; Mikule et al., 2003). The Triton X-100-soluble fraction (S) of growth cones, enriched in cytosolic and membrane-bound proteins, was separated from the insoluble pellet (P), enriched in cytoskeletal proteins (Haataja et al., 2002). By immunoblot, Neurabin I was observed in total brain and in the cytoskeletal enriched P fraction of the growth cones, whereas it was barely detectable in the cytosolic S fraction (Figure 5A). This result is consistent with the F-actin binding property of Neurabin I and its association with cytoskeletal proteins. Conversely, anti-Rac3 immunoprecipitations revealed the presence of Rac3 both in the P and S fractions of neuronal growth cones (Figure 5B). Altogether, these results indicate that Neurabin I and Rac3 are present in the growth cones of embryonic rat neurons and that they seemed to cofractionate with cytoskeletal proteins.
Figure 5.
Rac3 and Neurabin I are detectable in growth cones of rat brain and Neurabin I accumulates to the cytoskeleton upon FlagRac3 expression. (A) Growth cones were purified from embryonic rat brains and fractionated in Triton X-100-soluble cytosolic-enriched fraction (S) and Triton X-100-insoluble pellet (P), which is enriched of cytoskeletal proteins. Anti-Neurabin I immunoblot shows the 180-kDa band of Neurabin I (arrowhead) in total brain extract and in the P fraction of the growth cones. (B) Anti-Rac3 or control immunoprecipitations of the aforementioned extracts were resolved on SDS-PAGE, and endogenous Rac3 was revealed by anti-Rac3 immunoblot. (C) SK-N-BE cells transiently expressing wild-type or mutated FlagRac3 proteins were lysed. Total lysates were separated in S and P fractions as described above and analyzed in immunoblot with the indicated antibodies (anti-Neurabin I). Note that the expression of FlagRac3V12, FlagRac3N17 and FlagRac3 proteins increases the amount of 180-kDa Neurabin I (arrowhead) in the P fractions compared with untransfected cells. A concomitant reduction of Neurabin I levels in the corresponding S fractions is also evident. No variation is observed in FlagRac3ΔC-ter transfected cells. The levels of the Flagged-recombinant proteins (anti-FLAG) are shown as a control of transfection. Anti-integrin β1 immunoblot reveals the absence of membrane bound proteins in the P fractions, whereas anti-β-actin and anti-α-tubulin immunoblots confirm the presence of cytoskeletal proteins in the Triton X-100-insoluble fractions.
Rac3 Induces the Accumulation of Neurabin I to the Cytoskeleton
To investigate whether Rac3 may influence the cellular distribution of Neurabin I, FlagRac3-, FlagRac3V12-, and FlagRac3N17-transfected cells were lysed. The total lysate was fractionated in Triton X-100 soluble (S) and insoluble pellet (P). The quality of the two fractions was assayed with anti-integrin β1, which detects a specific signal only in the S fraction (Figure 5C). Anti-Neurabin I immunoblot showed that the amount of Neurabin I protein in the P fractions of transfected cells increased compared with untransfected cells, whereas it decreased in the corresponding cytosolic and membrane enriched fraction (S) (Figure 5C). On the contrary, no significant changes were observed in FlagRac3ΔC-ter-transfected cell extracts. FlagRac3, FlagRac3V12, and FlagRac3N17 proteins were all detected in the P fractions, whereas FlagRac3ΔC-ter was not. These results suggest that Rac3, either in its active or inactive forms, induces Neurabin I to associate to the cytoskeleton. This event seems to require Rac3-membrane binding since Rac3ΔC-ter, despite its ability to bind to Neurabin I (Figure 2B), did not induce the association of Neurabin I to the cytoskeleton (Figure 5C).
A Functional Rac3 Is Required to Induce Neuritogenesis
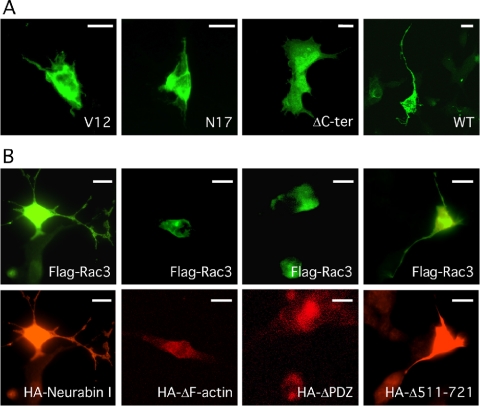
Because overexpression of FlagRac3 induced the formation and branching of long neurites (Albertinazzi et al., 1998; this study), we next investigated the ability of Rac3 mutants to stimulate neuritogenesis (counting as neurites only the projections departing from the soma and longer than one cell body) (Figures 6, 7, 8). FlagRac3-expressing cells presented an average of 2.4 neurites per cell. These neurites were highly branched with lengths up to ninefold the diameter of the cell body. By contrast, no neurite extension was observed in FlagRac3V12-, FlagRac3N17-, or FlagRac3ΔC-ter-expressing cells (Figure 6A), which presented a cellular shape similar to untransfected cells or to control cells transfected with an enhanced green fluorescent protein (EGFP) expression vector (Figure 7A). Control cells showed an average of 0.2 neurites per cell, never longer than 1.5-fold the diameter of the cell body (Figures 7A and 8). These results show that Rac3-induced neurite extension requires Rac3-membrane binding and GTPase activity.
Figure 6.
Rac3-induced neuritogenesis requires the GTPase activity and the membrane binding of Rac3, whereas it is inhibited by the overexpression of HANeurabin I lacking the F-actin or the PDZ domains. (A) FITC-conjugated anti-FLAG immunofluorescence on SK-N-BE cells transfected with FlagRac3V12 (V12), FlagRac3N17 (N17), FlagRac3ΔC-ter (ΔC-ter), or FlagRac3 (WT) and analyzed by confocal microscopy. Note that only wild-type FlagRac3 stimulates the extension of long and branched neurites. (B) FlagRac3 vector (FlagRac3) was cotransfected in SK-N-BE cells together with the indicated HANeurabin I vectors coding for wild type (HANeurabin I) or F-actin (HAΔF-actin), PDZ (HAΔPDZ), or coiled-coil domain (HAΔ511-721) deletion mutants. The same fields of cells immunostained with FITC-conjugated anti-FLAG (top) and with TRITC-conjugated anti-HA antibodies (bottom) are shown. Note that only wild-type HANeurabin I and HANeurabin I Δ511-721 allow the FlagRac3-induced neuritogenesis. Bar, 10 μm.
Figure 7.
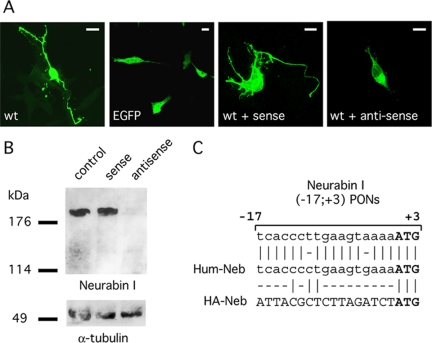
Rac3-induced neuritogenesis is mediated by Neurabin I. (A) FlagRac3 expressing SK-N-BE cells were incubated alone (wt) or with sense (wt + sense) or antisense (wt + antisense) Neurabin I (-17; +3) PONs. FITC-conjugated anti-FLAG immunofluorescence reveals long and branched neurites in FlagRac3-positive cells incubated without any treatment (wt) or with sense Neurabin I (-17; +3) PONs (WT + sense). Conversely, a strong inhibition of Rac3-dependent neuritogenesis is observed upon incubation with antisense Neurabin I (-17; +3) PONs (WT + antisense). Green fluorescent protein (EGFP)-transfected cells show the morphology of undifferentiated control cells. Bar, 10 μm. (B) Anti-Neurabin I immunoblot on whole cell lysates of SK-N-BE cells incubated with sense or antisense Neurabin I (-17; +3) PONs (top). Anti α-tubulin immunoblot is to ensure equal protein loading (bottom). (C) The alignment shows the homology of Neurabin I (-17; +3) PONs between nucleotide -17 and the start codon of the Rat Neurabin I cDNA, with the human wild type (Hum-Neb) or with the HA-tagged (HA-Neb) Neurabin I.
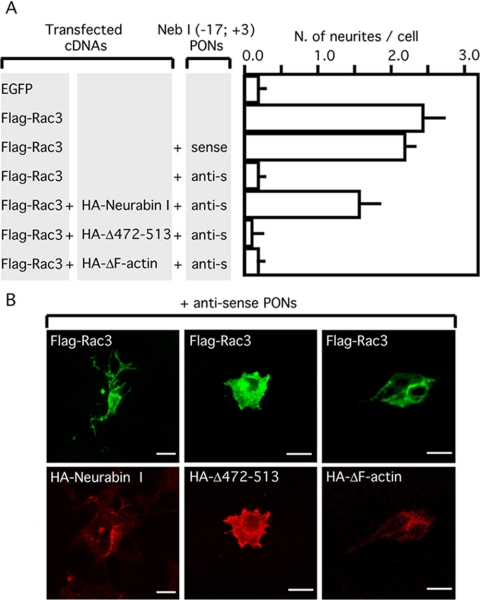
Figure 8.
Rescue of neuritogenesis by ectopic expression of HANeurabin I in the presence of antisense Neurabin I (-17; +3) PONs. SK-N-BE cells expressing FlagRac3 alone or in combination with HANeurabin I, HANeurabin I Δ472-513 or HANeurabin I ΔF-actin were incubated alone, with sense or antisense Neurabin I (-17; +3) PONs. EGFP-positive control cells were also assayed under the same conditions. (A) The histogram shows the average and the SD of at least three independent experiments. Only neurites departing from the soma and longer than one diameter of the cell body were counted. Note that the ectopic expression of Neurabin I overcomes the inhibition of FlagRac3-induced neuritogenesis mediated by antisense Neurabin I (-17; +3) PONs. Conversely, Neurabin I mutants, lacking the Rac3-binding or the F-actin domains fail to restore the neuritogenesis. (B) The immunofluorescence staining shows SK-N-BE cells transfected as described above and incubated with antisense Neurabin I (-17; +3) PONs. TRITC-conjugated anti-HA reveals the expression of HANeurabin I, HANeurabin I Δ472-513 or HANeurabin I ΔF-actin proteins (bottom photograms); the same fields stained with FITC-conjugated anti-FLAG reveal the expression of FlagRac3 (top photograms). Bar, 10 μm.
Next, the ability of FlagRac3 to induce neuritogenesis was tested in the presence of HANeurabin I or HANeurabin I mutants by cotransfection experiments. As shown in Figure 6B, FlagRac3 induced neuritogenesis in the presence of wild-type HANeurabin I, but it failed in cells expressing HANeurabin I mutants lacking the F-actin binding domain (HANeurabin IΔ1-144) or the PDZ domain (HANeurabin IΔ513-595). These mutants probably behave as dominant negative molecules that affect the F-actin or the PDZ binding properties of Neurabin I by forming dimers with the endogenous protein. As such, the HANeurabin IΔ511-721 mutant in which the dimerization ability is severely impaired by the deletion of the majority of the coiled-coil domains, did not compromise the Rac3-induced neuritogenesis. Thus, the presence of Neurabin I is required by Rac3 to induce neuritogenesis, and notably Neurabin I dimers need functional F-actin binding domain and PDZ domain to allow the Rac3-induced neuritogenesis.
Rac3-induced Neuritogenesis Requires Neurabin I
The ability of a functional Rac3 to stimulate neuritogenesis, the biochemical demonstration of an interaction between Rac3 and Neurabin I, the existence of Rac3/Neurabin I colocalization at the growth cone, and the Rac3-induced accumulation of Neurabin I to the cytoskeleton led us to investigate to what extent Neurabin I is important for Rac3-induced neuritogenesis. To this purpose, NB cells were transfected with FlagRac3 and treated with antisense or control sense rat Neurabin I (-17, +3) PONs. The Neurabin I (-17, +3) PONs were described previously to inhibit neuritogenesis in retinal ganglion cells (Nakanishi et al., 1997). The treatment of NB cells with antisense PONs caused a drop in Neurabin I levels, as observed by anti-Neurabin I immunoblot (Figure 7B). More importantly, treated cells showed strong reductions in the number and in the length of neurites of FlagRac3-positive cells, displaying a shape similar to control cells (Figure 7). On the contrary, the expression of Neurabin I and the neuritogenesis induced by FlagRac3 were not affected by sense PONs. Furthermore, a rescue of the neuritogenesis inhibited by antisense PONs was assayed. To this aim, we took the advantage of the antisense Neurabin I (-17; +3) PONs that abrogated the expression of endogenous Neurabin I but not of exogenous HANeurabin I carrying the HA-tag sequence in frame and upstream of Neurabin I initiation codon (Figures 7C). Thus, cells were cotransfected with FlagRac3 and HANeurabin I and treated with antisense or control sense Neurabin I PONs. In parallel, the HANeurabin I Δ472-513 mutant, lacking the Rac3 binding domain, was also assayed to investigate whether the Rac3/Neurabin I interaction is required. As shown in Figure 8, HANeurabin I was able to rescue neuritogenesis, whereas HANeurabin I Δ472-513 failed. The rescue of cell differentiation showed an average of 1.6 neurites per cell, slightly lower than that measured in FlagRac3-expressing cells (Figure 8). The difference probably depends on the expression level of HANeurabin I, because cells strongly stained by anti-HA antibody showed fully differentiated morphology. Notably, antisense PONs seemed to specifically target Neurabin I because the expression of Neurabin I cDNA is per se sufficient to overcome the inhibition of neuritogenesis. As observed for the Rac3-binding region, the F-actin binding domain is also required for the Rac3-induced neuritogenesis, because the HANeurabin I Δ1-144 mutant failed to rescue neuritogenesis inhibited by antisense PONs (Figure 8). In summary, these results indicate that Rac3-induced neurite outgrowth requires Rac3/Neurabin I interaction, the presence of the complex on the actin filaments, and supports the hypothesis that Neurabin I is significant in Rac3-dependent neuritogenesis.
DISCUSSION
Rac3 is a neuronal protein that belongs to the large family of Rho GTPases, capable of inducing neuritogenesis when overexpressed in developing neurons (see Introduction). In the present study, we demonstrated, through the yeast two-hybrid approach, that the neuronal F-actin binding protein Neurabin I is a partner of Rac3. More importantly, we demonstrated that Rac3 interacts with Neurabin I in neurons and neuroblastoma cells, inducing its accumulation to the cytoskeleton; that Rac3 and Neurabin I spatially coexist at the site of neurite expansion, growth cones; and that Rac3 requires the binding to Neurabin I to promote neuritogenesis. In mechanistic terms, we show that the Rac3/Neurabin I interaction occurs regardless of the activity of Rac3 (Table 1 and Figures 2 and 3). This observation rules out a possible function for Neurabin I as an effector protein that would only interact with active Rac3 and also as a GEF regulator. GEF proteins, indeed, bind to nucleotide-free and GDP-loaded Rac but not to GTP-Rac (Meller et al., 2002). Similarly, because Neurabin I does not contain a GAP domain or GDI conserved residues, it is unlikely that Neurabin I acts as a GAP or GDI regulator. Therefore, we envisage Neurabin I as a novel type of Rac-interacting molecule, capable of binding to Rac3 during active and inactive states.
We observed that Rac3 induces an accumulation of Neurabin I to the cytoskeleton (Figure 5). This is consistent with previous work showing the localization of the Neurabin I-related protein Spinophilin/Neurabin II (Allen et al., 1997; Satoh et al., 1998) to F-actin, in a Rac1-dependent manner (Stephens and Banting, 2000). Regardless, we found that the accumulation of Neurabin I to the cytoskeleton by Rac3 occurs independently from the occurrence of neurite extension (it occurs in cells expressing Rac3V12 and Rac3N17) but is highly dependent on the membrane binding state of Rac3. This suggests that an essential and early step in Rac3-induced neuritogenesis is the proper localization of the complex. It is not difficult to imagine that at this point other proteins will enter into play, such as the integrin-binding protein CIB that specifically interacts with the C-terminal tail of Rac3 (Haataja et al., 2002). In contrast, the observation that the accumulation of Neurabin I to the cytoskeleton is required for Rac3 to induce neuritogenesis is demonstrated by 1) the lack of neurite outgrowth in Rac3 cells coexpressing the Neurabin I mutant lacking the F-actin binding domain (Figure 6B) and 2) by the failure of this mutant to rescue neuritogenesis in Rac3 expressing/Neurabin I antisense cells (Figure 8). Although the full molecular machinery involved in neuritogenesis is large and complex, the results presented here indicate that one such mechanism involves Neurabin I-mediated engagement of Rac3 and other proteins on actin filaments. In fact, the expression of the Neurabin I mutant lacking the PDZ domain interfering with Rac3-induced neuritogenesis (Figure 6B) suggests that among such proteins are those with capacity to bind to the Neurabin I-PDZ domain.
It was shown that the PDZ domain of Neurabin I binds to the serine/threonine protein kinase p70S6 (Burnett et al., 1998), which is a known effector of Rac1 (Chou and Blenis, 1996). Interestingly, Rac3 interacts with the protein region of Neurabin I next to the PDZ domain, between a.a. 472 and 513 (Figure 3). Therefore, Neurabin I might be a bridge that holds together, in proximity, Rac3 and putative effector proteins. Neurabin I also binds to other proteins such as TGN38 and PP1 (Stephens and Banting, 1999; Terry-Lorenzo et al., 2002); the closely related Spinophilin/Neurabin II binds to Tiam1, a GEF protein that activates Rac (Buchsbaum et al., 2003); and both Neurabin I and Spinophilin/Neurabin II bind to the Rho-specific GEF protein Lcf (Ryan et al., 2005). These data strengthen the idea of Neurabins as scaffold molecules that congregate proteins acting on the Rac/Rho signaling cascade. As shown by the cellular distributions of Rac3 and Neurabin I, the protein complex is present at the tip of extending neurites, where it colocalizes with F-actin (Figure 4). Thus, we propose Neurabin I as an anchor that recruits a fraction of Rac3 and Rac3-interacting proteins close to F-actin, at the growth cone.
We observed that the GTPase activity of Rac3 is essential for the Rac3-induced neuritogenesis (Figure 6A), because it is used to transduce the neuritogenic signaling to downstream proteins. Because Rac proteins can induce the formation of lamellipodia (Meyer and Feldman, 2002), an accumulation of Rac3 within the growth cones might contribute to the protrusion or the branching of neurites. By contrast, the knockdown of Neurabin I expression by antisense PONs or a failure of interaction between Neurabin I and Rac3 interferes with the neuritogenesis induced by Rac3, probably because it interferes with the formation of this protein complex.
Several reports showed that Neurabin I and Spinophilin/Neurabin II are implicated in dendritic spine morphogenesis: one Neurabin I mutant truncated at residue 278, stimulates the F-actin polymerization in dendritic spines and influences the spine motility (Oliver et al., 2002; Zito et al., 2004), whereas Spinophilin/Neurabin II knockout mice present increased spine densities (Feng et al., 2000). These studies reveal a function for Neurabin proteins in regulating spine motility that facilitates synapse formation and synaptic plasticity. A recent report has shown that the binding of the Rho-specific GEF protein Lfc to Neurabin is required to regulate the morphology of dendritic spines (Ryan et al., 2005) and that the overexpression of Lfc in hippocampal neurons reduces the number and the branching of dendrites, because of the activation of Rho. It is the current view that Rho proteins limit the neurite protrusion and mediate growth cone collapse, whereas Rac proteins induce neurite extension. These data further strengthen the view that Neurabin proteins mediate the Rho/Rac signaling events, which regulate cytoskeletal rearrangements during spine morphology and neuritogenesis. Our manuscript suggests a novel function for Neurabin I in Rac3-induced neuritogenesis, implicating that spine motility and neuritogenesis might relay on similar molecular mechanisms. The Rac3 interaction with Neurabin I is as essential as the Rac3-GTPase activity and the Rac3 membrane targeting for triggering a signaling cascade involved in lamellipodia formation and neurite extension.
Acknowledgments
We are grateful to E. Cassin for the immunofluorescence experiments on primary neurons; Y. Takai, S. Elledge, and I. de Curtis for reagents; P. Vaghi (Centro Grandi Strumenti-Universita' di Pavia) for confocal images; F. Casagranda, E. Gherardi, and C. Murphy for critical reading the manuscript; and G. Biamonti and the people in the laboratory for helpful discussions. D. O. was partially supported by a European Molecular Biology Organization fellowship, by Fondazione Telethon Onlus (459/bi), and by Ministero dell'Istruzione, dell'Universitá e della Ricerca/Fondo per gli Investimenti della Ricerca di Base (RBNE01RNN7). We are grateful to Fondazione Cariplo for financial support of M.S.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0753) on March 8, 2006.
Abbreviations used: NB, neuroblastoma; PDZ, PSD-95, DlgA, ZO-1-like domain; PON, phosphorothioate oligonucleotide; RA, retinoic acid.
References
- Abemayor, E., and Sidell, N. (1989). Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ. Health Perspect. 80, 3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertinazzi, C., Gilardelli, D., Paris, S., Longhi, R., and de Curtis, I. (1998). Overexpression of a neural-specific rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J. Cell Biol. 142, 815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, P. B., Ouimet, C. C., and Greengard, P. (1997). Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. USA 94, 9956-9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenstrom, P. (1999). Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11, 95-102. [DOI] [PubMed] [Google Scholar]
- Bolis, A., Corbetta, S., Cioce, A., and de Curtis, I. (2003). Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: implications for a role of Rac3 in Purkinje cell differentiation. Eur. J. Neurosci. 18, 2417-2424. [DOI] [PubMed] [Google Scholar]
- Bruckner, K., Pablo Labrador, J., Scheiffele, P., Herb, A., Seeburg, P. H., and Klein, R. (1999). EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511-524. [DOI] [PubMed] [Google Scholar]
- Buchsbaum, R. J., Connolly, B. A., and Feig, L. A. (2003). Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J. Biol. Chem. 278, 18833-18841. [DOI] [PubMed] [Google Scholar]
- Burnett, P. E., Blackshaw, S., Lai, M. M., Qureshi, I. A., Burnett, A. F., Sabatini, D. M., and Snyder, S. H. (1998). Neurabin is a synaptic protein linking p70 S6 kinase and the neuronal cytoskeleton. Proc. Natl. Acad. Sci. USA 95, 8351-8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, M. M., and Blenis, J. (1996). The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell 85, 573-583. [DOI] [PubMed] [Google Scholar]
- da Silva, J. S., and Dotti, C. G. (2002). Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3, 694-704. [DOI] [PubMed] [Google Scholar]
- Del Pozo, M. A., Kiosses, W. B., Alderson, N. B., Meller, N., Hahn, K. M., and Schwartz, M. A. (2002). Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4, 232-239. [DOI] [PubMed] [Google Scholar]
- Dickson, B. J. (2001). Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11, 103-110. [DOI] [PubMed] [Google Scholar]
- Driessens, M. H., Hu, H., Nobes, C. D., Self, A., Jordens, I., Goodman, C. S., and Hall, A. (2001). Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr. Biol. 11, 339-344. [DOI] [PubMed] [Google Scholar]
- Feng, J., Yan, Z., Ferreira, A., Tomizawa, K., Liauw, J. A., Zhuo, M., Allen, P. B., Ouimet, C. C., and Greengard, P. (2000). Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA 97, 9287-9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini, D., Colaluca, I. N., Vodermaier, H. C., Biamonti, G., Giacca, M., Falaschi, A., Riva, S., and Peverali, F. A. (2003). Early mitotic degradation of the homeoprotein HOXC10 is potentially linked to cell cycle progression. EMBO J. 22, 3715-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger, E. (2002). How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation 70, 385-396. [DOI] [PubMed] [Google Scholar]
- Goslin, K., and Banker, G. (1991). Rat hypocampal neurons in low-density culture. In: Culturing Nerve Cells, ed. K.G.a.G. Banker, Cambridge, MA: Massachusetts Institute of Technology Press, 251-281.
- Haataja, L., Groffen, J., and Heisterkamp, N. (1997). Characterization of RAC3, a novel member of the Rho family. J. Biol. Chem. 272, 20384-20388. [DOI] [PubMed] [Google Scholar]
- Haataja, L., Kaartinen, V., Groffen, J., and Heisterkamp, N. (2002). The small GTPase Rac3 interacts with the integrin-binding protein CIB and promotes integrin alpha(IIb)beta(3)-mediated adhesion and spreading. J. Biol. Chem. 277, 8321-8328. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Henkemeyer, M., Orioli, D., Henderson, J. T., Saxton, T. M., Roder, J., Pawson, T., and Klein, R. (1996). Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86, 35-46. [DOI] [PubMed] [Google Scholar]
- Lamarche-Vane, N., and Hall, A. (1998). CdGAP, a novel proline-rich GTPase-activating protein for Cdc42 and Rac. J. Biol. Chem. 273, 29172-29177. [DOI] [PubMed] [Google Scholar]
- Lohse, K., Helmke, S. M., Wood, M. R., Quiroga, S., de la Houssaye, B. A., Miller, V. E., Negre-Aminou, P., and Pfenninger, K. H. (1996). Axonal origin and purity of growth cones isolated from fetal rat brain. Brain Res. Dev. Brain Res. 96, 83-96. [DOI] [PubMed] [Google Scholar]
- Luo, L. (2000). Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173-180. [DOI] [PubMed] [Google Scholar]
- Luo, L., Jan, L., and Jan, Y. N. (1996). Small GTPases in axon outgrowth. Perspect. Dev. Neurobiol. 4, 199-204. [PubMed] [Google Scholar]
- Malosio, M. L., Gilardelli, D., Paris, S., Albertinazzi, C., and de Curtis, I. (1997). Differential expression of distinct members of Rho family GTP-binding proteins during neuronal development: identification of Rac1B, a new neural-specific member of the family. J. Neurosci. 17, 6717-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, N., Irani-Tehrani, M., Kiosses, W. B., Del Pozo, M. A., and Schwartz, M. A. (2002). Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat. Cell Biol. 4, 639-647. [DOI] [PubMed] [Google Scholar]
- Meyer, G., and Feldman, E. L. (2002). Signaling mechanisms that regulate actin-based motility processes in the nervous system. J. Neurochem. 83, 490-503. [DOI] [PubMed] [Google Scholar]
- Mikule, K., Sunpaweravong, S., Gatlin, J. C., and Pfenninger, K. H. (2003). Eicosanoid activation of protein kinase C epsilon: involvement in growth cone repellent signaling. J. Biol. Chem. 278, 21168-21177. [DOI] [PubMed] [Google Scholar]
- Muly, E. C., Allen, P., Mazloom, M., Aranbayeva, Z., Greenfield, A. T., and Greengard, P. (2004). Subcellular distribution of neurabin immunolabeling in primate prefrontal cortex: comparison with spinophilin. Cereb. Cortex 14, 1398-1407. [DOI] [PubMed] [Google Scholar]
- Nakanishi, H., Obaishi, H., Satoh, A., Wada, M., Mandai, K., Satoh, K., Nishioka, H., Matsuura, Y., Mizoguchi, A., and Takai, Y. (1997). Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J. Cell Biol. 139, 951-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, C. J., Terry-Lorenzo, R. T., Elliott, E., Bloomer, W. A., Li, S., Brautigan, D. L., Colbran, R. J., and Shenolikar, S. (2002). Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Mol. Cell. Biol. 22, 4690-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli, D., Henkemeyer, M., Lemke, G., Klein, R., and Pawson, T. (1996). Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 15, 6035-6049. [PMC free article] [PubMed] [Google Scholar]
- Orioli, D., and Klein, R. (1997). The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 13, 354-359. [DOI] [PubMed] [Google Scholar]
- Peverali, F. A., Orioli, D., Tonon, L., Ciana, P., Bunone, G., Negri, M., and Della-Valle, G. (1996). Retinoic acid-induced growth arrest and differentiation of neuroblastoma cells are counteracted by N-myc and enhanced by max overexpressions. Oncogene 12, 457-462. [PubMed] [Google Scholar]
- Pfenninger, K. H., Ellis, L., Johnson, M. P., Friedman, L. B., and Somlo, S. (1983). Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell 35, 573-584. [DOI] [PubMed] [Google Scholar]
- Ryan, X. P., Alldritt, J., Svenningsson, P., Allen, P. B., Wu, G. Y., Nairn, A. C., and Greengard, P. (2005). The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron 47, 85-100. [DOI] [PubMed] [Google Scholar]
- Saras, J., and Heldin, C. H. (1996). PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem. Sci. 21, 455-458. [DOI] [PubMed] [Google Scholar]
- Satoh, A., et al. (1998). Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J. Biol. Chem. 273, 3470-3475. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., and Hall, A. (2002). Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587-1609. [DOI] [PubMed] [Google Scholar]
- Seabra, M. C. (1998). Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal 10, 167-172. [DOI] [PubMed] [Google Scholar]
- Shamah, S. M., et al. (2001). EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233-244. [DOI] [PubMed] [Google Scholar]
- Song, H., and Poo, M. (2001). The cell biology of neuronal navigation. Nat. Cell Biol. 3, E81-E88. [DOI] [PubMed] [Google Scholar]
- Stephens, D. J., and Banting, G. (1999). Direct interaction of the trans-Golgi network membrane protein, TGN38, with the F-actin binding protein, neurabin. J. Biol. Chem. 274, 30080-30086. [DOI] [PubMed] [Google Scholar]
- Stephens, D. J., and Banting, G. (2000). In vivo dynamics of the F-actin-binding protein neurabin-II. Biochem. J. 345 2, 185-194. [PMC free article] [PubMed] [Google Scholar]
- Symons, M., and Settleman, J. (2000). Rho family GTPases: more than simple switches. Trends Cell Biol. 10, 415-419. [DOI] [PubMed] [Google Scholar]
- Tanaka, E., and Sabry, J. (1995). Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell 83, 171-176. [DOI] [PubMed] [Google Scholar]
- Terry-Lorenzo, R. T., Carmody, L. C., Voltz, J. W., Connor, J. H., Li, S., Smith, F. D., Milgram, S. L., Colbran, R. J., and Shenolikar, S. (2002). The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. J. Biol. Chem. 277, 27716-27724. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and Goodman, C. S. (1996). The molecular biology of axon guidance. Science 274, 1123-1133. [DOI] [PubMed] [Google Scholar]
- Van Aelst, L., and D'Souza-Schorey, C. (1997). Rho GTPases and signaling networks. Genes Dev. 11, 2295-2322. [DOI] [PubMed] [Google Scholar]
- Wahl, S., Barth, H., Ciossek, T., Aktories, K., and Mueller, B. K. (2000). Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149, 263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, T. W., and Bargmann, C. I. (2001). Dynamic regulation of axon guidance. Nat. Neurosci. 4, 1169-1176. [DOI] [PubMed] [Google Scholar]
- Zito, K., Knott, G., Shepherd, G. M., Shenolikar, S., and Svoboda, K. (2004). Induction of spine growth and synapse formation by regulation of the spine actin cytoskeleton. Neuron 44, 321-334. [DOI] [PubMed] [Google Scholar]