Abstract
Both phospholipase (PL) C-γ1 and Akt (protein kinase B; PKB) are signaling proteins that play significant roles in the intracellular signaling mechanism used by receptor tyrosine kinases, including epidermal growth factor (EGF) receptor (EGFR). EGFR activates PLC-γ1 directly and activates Akt indirectly through phosphatidylinositol 3-kinase (PI3K). Many studies have shown that the PLC-γ1 pathway and PI3K–Akt pathway interact with each other. However, it is not known whether PLC-γ1 binds to Akt directly. In this communication, we identified a novel interaction between PLC-γ1 and Akt. We demonstrated that the interaction is mediated by the binding of PLC-γ1 Src homology (SH) 3 domain to Akt proline-rich motifs. We also provide a novel model to depict how the interaction between PLC-γ1 SH3 domain and Akt proline-rich motifs is dependent on EGF stimulation. In this model, phosphorylation of PLC-γ1 Y783 by EGF causes the conformational change of PLC-γ1 to allow the interaction of its SH3 domain with Akt proline-rich motifs. Furthermore, we showed that the interaction between PLC-γ1 and Akt resulted in the phosphorylation of PLC-γ1 S1248 by Akt. Finally, we showed that the interaction between PLC-γ1 and Akt enhanced EGF-stimulated cell motility.
INTRODUCTION
The stimulatory effects of receptor tyrosine kinases (RTKs) on cell growth are mediated by downstream signaling proteins. Phospholipase (PL) C-γ1 is one of these signaling proteins and plays a significant role in the intracellular signaling mechanism used by RTKs (Wahl and Carpenter, 1991). PLC-γ1 has been implicated in many growth factor (GF)-induced cell signaling processes, including cell proliferation, differentiation, receptor endocytosis, cell motility, membrane ruffle formation, and branching tubulogenesis (Wahl and Carpenter, 1991; Kamat and Carpenter, 1997; Wells et al., 1998; Gual et al., 2000; Bivona et al., 2003; Chou et al., 2003; Meyer et al., 2003; Choi et al., 2004). Overexpression and hyperactivation of PLC-γ1 have been implicated in breast and prostate cancers. Especially, PLC-γ1 activity has been linked to cancer cell invasion (Thomas et al., 2003; Wells and Grandis, 2003).
PLC-γ1, a 145-kDa protein, contains two Src homology (SH) 2 domains, one SH3 domain, and two pleckstrin homology (PH) domains and catalyzes the hydrolysis of phosphatidylinositol-4,5-bisphosphate, creating inositol 1,4,5-triphosphate (IP3) and diacylglycerol. These second messengers are known to stimulate the release of Ca2+ from internal stores and to activate protein kinase C (PKC), respectively (Wahl and Carpenter, 1991). PLC-γ1 forms a complex in vivo with epidermal growth factor (EGF) receptor (EGFR) through its SH2 domain interaction (Wahl et al., 1988; Margolis et al., 1989, 1990; Meisenhelder et al., 1989; Anderson et al., 1990). Complex formation leads to phosphorylation of PLC-γ1 on tyrosine residues and an increase in its enzymatic activity (Kim et al., 1991; Ronnstrand et al., 1992; Rotin et al., 1992).
All of the PLC-γ1 domains have been implicated in regulating the cellular localization of PLC-γ1 and in regulating GF-induced cell signaling (Kamat and Carpenter, 1997; Wang and Moran, 2002; Wang and Wang, 2003). Recent studies have shown that PLC-γ1 is involved in much broader cell signaling than previously revealed. Interestingly, most of the recently identified interactions between PLC-γ1 and its binding proteins are mediated by its SH3 domain. EGF stimulates the interaction between PLC-γ1 and PLD2 to potentiate EGF-induced IP3 formation and Ca2+ increase. The interaction between PLC-γ1 and PLD2 is mediated by PLC-γ1 SH3 domain (Jang et al., 2003). PLC-γ1 is essential for the activation of calcium entry into cells after stimulation on cell surface receptors, and PLC-γ1 SH3 domain was required for this effect (Patterson et al., 2002). PLC-γ1 SH3 domain acts as a guanine nucleotide exchange factor (GEF) for PIKE to regulate nerve growth factor-induced cell mitogenesis (Ye et al., 2002). PLC-γ1 SH3 domain acts as a GEF for dynamin-1 to regulate EGFR endocytosis, and the interaction between PLCγ-1 SH3 domain and dynamin-1 is EGF dependent (Choi et al., 2004).
It has been reported that PLC-γ1 is required for cell motility induced by many GFs, including EGF (Chen et al., 1994), platelet-derived growth factor (Kundra et al., 1994), and hepatocyte growth factor (Derman et al., 1996). Although the mechanisms by which PLC-γ1 regulates cell motility are not clear yet, it was suggested that activation of PLC-γ1 by EGF results in the reorganization of cytoskeleton (Wells et al., 2002). The role of PLC-γ1 in the regulation of cell motility has been shown in a variety of cell types, especially carcinoma cells (Wells et al., 2002).
Akt (protein kinase B; PKB) is a central player in the signal transduction pathways activated in response to GFs and is thought to contribute to several cellular functions, including nutrient metabolism, cell growth, and apoptosis. Alteration of Akt activity is associated with several human diseases, including cancer and diabetes (Hanada et al., 2004). Akt is composed of an amino terminal PH domain, a central kinase domain, and a carboxyl terminal regulatory domain. All three Akt isoforms have a carboxy-terminal extension of ∼40 amino acids. This region possesses the FXXF/Y-S/T-Y/F hydrophobic motif. In mammalian Akt isoforms, this motif is identical (FPQFSY). Deletion of this motif abolishes the enzymatic activity of Akt (Andjelkovic et al., 1997). GFs activate Akt by activating phosphatidylinositol 3-kinase (PI3K) (Hanada et al., 2004). GF-induced phosphorylation of T308 and S473 is essential for Akt activity. Point mutants at these sites with Ala show little activity, and the phosphorylation-mimicking mutants (T308D/S473D) show constitutive kinase activity (Alessi et al., 1996). It was recently reported that Akt could be activated by the phosphorylation of its tyrosine residues, including Y315 and Y326. These sites were phosphorylated by c-Src and mediated by the interaction of Src SH3 domain with the PXXP motif in the C-terminal of Akt in an EGF-dependent manner (Chen and Wang, 2001). The minimal substrate consensus sequence for Akt is RXRXXS/T. The best-defined function of Akt in GF-induced cell signaling is to regulate apoptosis. Akt promotes cell survival by phosphorylating BAD S136 (Datta et al., 1997; del Peso et al., 1997). Recently, Akt is also suggested to regulate cancer cell invasion and cell motility (Firtel and Chung, 2000).
It is well established that activated EGFR directly stimulates PLC-γ1 by phosphorylating its tyrosine residues and indirectly stimulate Akt by activating PI3K (Carpenter and Ji, 1999). Both PLC-γ1 and PI3K–Akt pathways play significant role in EGFR-mediated cell signaling. Although both PLC-γ1 and PI3K–Akt play distinct role in cell signaling, the two pathways have been shown to interact each other. For example, PLC-γ1 and PI3K are both involved in lipid metabolism and use the same substrate (phosphatidylinositol bisphosphate; PIP2) (Carpenter and Ji, 1999). Both PLC-γ1 and PI3K–Akt pathways are involved in cell mitogenesis and motility (Chen et al., 1994). It is known that PLC-γ1 may regulate Ras activity through the activation of rasGRP and activated Ras then stimulates the activation of PI3K (Ehrhardt et al., 2004). We have recently shown that PI3K may affect the translocation of PLC-γ1 by generating PI3P (Wang and Wang, 2003). However, it is not know whether PLC-γ1 directly binds to Akt. In this communication, we identified a novel interaction between PLC-γ1 and Akt. We demonstrated that the interaction is mediated by the binding of PLC-γ1 SH3 domain to Akt proline motifs. We also provide a novel model to depict how the interaction between PLC-γ1 SH3 domain and Akt proline motifs is dependent on EGF stimulation. In this model, phosphorylation of PLC-γ1 Y783 by EGF causes the conformational change of PLC-γ1 to allow the interaction of its SH3 domain with Akt proline motifs. Furthermore, we showed that the interaction between PLC-γ1 and Akt caused phosphorylation of PLC-γ1 S1248 and enhanced EGF-stimulated cell motility.
MATERIALS AND METHODS
Cell Culture, Treatment, and Transfection
COS-7 cells were grown at 37°C in DMEM containing 10% fetal bovine serum and were maintained in a 5% CO2 atmosphere. To specifically activate EGFR, COS-7 cells were serum starved for 24 h, and EGF was added to a final concentration of 100 ng/ml. COS-7 cells were transfected using standard calcium phosphate method with BES buffer (140 mM NaCl, 0.75 mM sodium phosphate dibasic [Na2HPO4], 25 mM BES, pH 6.95). To inhibit PI3K–Akt activation, cells were pretreated with 100 nM wortmannin for 10 min and then incubated with EGF in the continuous presence of 100 nM wortmannin. To inhibit PLC-γ1 activity, cells were pretreated with 10 μM U73122 for 30 min and then incubated with EGF in the continuous presence of 10 μM U73122. To inhibit PKA activity, cells were pretreated with 10 μM H89 or 500 nM KT5720 and then incubated with EGF in the continuous presence of 10 μM H89 or KT5720 (500 nM).
Antibodies and Chemicals
Glutathione-agarose beads coupled with glutathione S-transferase (GST)-Akt were purchased from Upstate Biotechnology (Lake Placid, NY). GST-Akt was eluted from glutathione-agarose beads in the glutathione elution buffer (10 mM glutathione and 50 mM Tris-HCl, pH 8.0). Rabbit anti-Akt was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-PLC-γ1 and anti-phosho-serine (1248) of PLC-γ1 were purchased from Upstate Biotechnology. Rabbit anti-phosphotyrosine (Y783) of PLC-γ1 was purchased from Medicore (Montreal, Quebec, Canada). Rabbit anti-GFP antibody was purchased from Clonetech (Mountain View, CA), and it can recognize both green fluorescent protein (GFP) and its derivatives, such as yellow fluorescent protein (YFP). Glutathione cross-linked to 4% agarose, goat anti-mouse IgG conjugated with agarose, and thrombin were purchased from Sigma-Aldrich (St. Louis, MO). Unless otherwise specified, all the chemicals were purchased from Sigma-Aldrich.
Plasmids
The YFP-tagged full-length and various deletion mutants of PLC-γ1 were generated previously (Wang and Wang, 2003). Several GST fusion proteins (including PLC-γ1N-SH2, PLC-γ1CSH2, PLC-γ1SH3, PLC-γ1NPH, and PLC-γ1SH2-SH2-SH3) were made previously in the laboratory (Wang et al., 1998). GFP-Akt was a gift from Dr. Julian Downward (Cancer Research UK London Research Institute, London, United Kingdom). All of the mutants with point mutation were created with the QuikChange multiple site-directed mutagenesis kit (Stratagene, La Jolla, CA) with YFP-tagged wild type PLC-γ1 (PLC-γ1) as template. These mutants include a YFP-tagged mutant PLC-γ1 with mutation of Y771 to phenylalanine (termed Y771F), a YFP-tagged mutant PLC-γ1 with mutation of Y783 to phenylalanine (termed Y783F), a YFP-tagged mutant PLC-γ1 with mutation of both Y771 and Y783 to phenylalanine (termed Y/Y), a YFP-tagged mutant PLC-γ1 with mutation of serine 1248 to alanine (termed S1248A), a GFP-tagged mutant Akt with mutation of P424 to alanine (P424A), a GFP-tagged mutant Akt with mutation of P476 to alanine (P467A), and a GFP-tagged mutant PLC-γ1 with mutation of both P424 and P476 to alanine (termed P/P).
Expression and Purification of GST Fusion PLC-γ1 N-SH2, C-SH2, SH3, SH2-SH2-SH3, and PH Domains
To purify NSH2, CSH2, SH3, and SH2-SH2-SH3 domains of PLC-γ1, the pGEX-3X plasmid containing the wild-type N-SH2, C-SH2, SH3, and SH2-SH2-SH3 domains of PLC-γ1 was transformed into Escherichia coli DH5α. Cells were grown to an optical density (OD)600 of 0.3–0.4 and induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 3 h at 37°C. After pelleting, cells were lysed by sonication in 50 mM Tris, pH 8.0, 100 mM NaCl, 10% glycerol, 1 mM dithiothreitol (DTT), containing protease inhibitors [0.02% NaN3, 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 μg/ml aprotinin, and 1 μM pepstatin A]. Triton X-100 was added to a final concentration of 1%, and particulates were removed by centrifugation for 10 min at 10,000 rpm in a JA-17 (Beckman Coulter, Fullerton, CA) rotor. The clarified lysate was incubated with glutathione-agarose beads (Sigma-Aldrich) for 1 h at 4°C, washed three times with ice-cold GST wash buffer containing 1 mM DTT plus protease inhibitors, and protein bound to the glutathione-agarose beads was stored at 4°C.
To purify PH domain of PLC-γ1, E. coli BL-21, a strain that is defective in OmpT and Lon protease production and expresses the fusion protein in a more soluble and intact form, were used for transformation. Cells were grown to an OD600 of 0.3–0.4 and induced with 0.1 mM IPTG for 8–10 h at 22°C. The later steps were the same as the other GST-mutants.
In Vitro Binding Assay
We first purified Akt from GST-Akt by thrombin cleavage. Thrombin was dissolved into 1× phosphate-buffered saline (PBS) to make the stock concentration at 200 U/ml. For each milliliter of bead, 50 μl of thrombin solution and 950 μl of 1× PBS were added into the solution and mixed at room temperature for 2–16 h. After centrifuge at 500 × g for 5 min to pellet the beads, the supernatant containing the Akt was collected for the in vitro GST pull-down experiments. For the in vitro binding assay, GST and GST-PLC-γ1SH3 beads were incubated with purified Akt for 30 min. After washing three times, the final precipitates were blotted with anti-GST and anti-Akt antibodies.
GST Fusion Protein Pull-Down Experiment
COS-7 cells were treated with or without EGF and or wortmannin and then lysed and scraped into 0.5 ml of BOS buffer (50 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1% NP-40, 10% glycerol, 10 mM NaF, 2.5 mM MgCl2, and 1 mM EDTA) with protease inhibitor. The lysates were centrifuged at 21,000 × g at 4°C for 30 min. Supernatants were used in the binding assay. GST fusion PLC-γ1 proteins bound to glutathione-agarose-beads in BOS buffer were added and incubated at 4°C for 1 h. Beads were collected by centrifugation, washed three times with BOS buffer, and then loading buffer was added. The pull-down proteins were analyzed by immunoblotting with anti-GST and anti-Akt antibodies.
Subcellular Fractionation and Total Cell Lysates
Isolation of plasma membrane (PM), endosomal (EN), and cytosolic (Cyt) fractions was carried out by our previously described method (Wang et al., 1999). Briefly, after treatment, cells were scraped into homogenization buffer [0.25 M sucrose, 20 mM Tris-HCl, pH 7, 1 mM MgCl2, 4 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 μg/ml aprotinin, and 1 μM pepstatin A] and homogenized. The homogenates were centrifuged at 200 × g for 5 min to remove cell debris and nuclei (p1). The postnuclear supernatant (S1) was then centrifuged at 1500 × g for 10 min to yield a supernatant (S2) and a pellet (P2). Next, P2 was resuspended in homogenization buffer (0.25 M sucrose), overlaid upon an equal volume of 1.42 M sucrose buffer, and centrifuged at 82,000 × g for 1 h. The pellicule at the 0.25–1.42 M interface was collected as the PM fraction. The S2 fraction was centrifuged at 100,000 × g for 30 min to yield the soluble Cyt fraction and a microsomal pellet. This pellet was resuspended in 0.25 M sucrose buffer and overlaid upon a discontinuous sucrose gradient containing equal volumes of homogenization buffer at 1.00 and 1.15 M sucrose. The resuspension was centrifuged at 200,000 × g for 1.5 h to obtain the purified EN fraction at the 0.25–1.00 M interface.
The crude subcellular fractionation experiments were carried out as described previously (Wang and Wang, 2003). Briefly, after treatment, cells were scraped into homogenization buffer and homogenized. The homogenates were centrifuged at 280 × g for 5 min to remove cell debris and nuclei. The postnuclear supernatant was centrifuged at 100,000 × g for 1 h to yield a supernatant (S100), which was collected as Cyt and a particulate (P100) fraction, and was dissolved in immunoprecipitation buffer and collected as total membrane (Mem). Immunoprecipitation experiments were performed as described below.
Immunoprecipitation
Immunoprecipitation experiments were carried out as described previously (Wang et al., 1999). Briefly, cells were homogenized and lysed with immunoprecipitation buffer [20 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% sodium deoxycholate, 100 mM NaF, 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 μg/ml aprotinin, and 1 μM pepstatin A] overnight at 4°C. Cell lysates were then centrifuged at 22,000 × g for 30 min to remove debris. The supernatants, containing 1 mg of total protein and precleared with the agarose beads, were used to incubate with 1 μg of specific antibody for 2 h with gentle mixing by inverting. Then, goat anti-mouse IgG conjugated with agarose or protein A conjugated with agarose was added to each fraction and incubated for 2 h with agitation. Finally, both the agarose beads and the nonprecipitated supernatant were collected by centrifugation. For the controls, mouse or rabbit IgG were used to replace the primary antibodies. The agarose beads were washed three times with immunoprecipitation buffer, and 1× loading buffer was added. The sample was boiled for 5 min and ready for Western blot.
Immunoblotting
Immunoblotting was performed as described previously (Wang et al., 1999). For the detection of early endosomal antigen (EEA)1, PLC-γ1, and Akt in each subcellular fraction of COS-7 cells, aliquots containing 1/10 of the protein from each fraction were used. For the detection of PLC-γ1 and Akt in the immunoprecipitates, 1/10 of the immunoprecipitate from each lysate was used. Protein samples were separated by electrophoresis through 8% polyacrylamide SDS-containing gels and electrophoretically transferred onto nitrocellulose filter paper. Filters were probed with the respective primary antibody. The primary antibodies were detected with a polyclonal goat anti-rabbit IgG coupled to horseradish peroxidase (HRP) or a polyclonal goat anti-mouse IgG coupled to HRP followed by enhanced chemiluminescence development (Pierce Chemical, Rockford, IL) and light detection with Fuji Super RX film (FujiFilm, Tokyo, Japan). Quantification of the results was achieved by using a FluorChem digital imaging system (Alpha Innotech, San Leandro, CA) and Total Lab software version 1.11 (Nonlinear Dynamics, Newcastle, United Kingdom).
Immunofluorescence
Indirect immunofluorescence was carried out as described previously (Wang et al., 1999). Cells were grown on glass coverslips to subconfluence and serum starved for 24 h. After treatment with 100 ng/ml EGF for the indicated time, the cells were fixed by immersion in –20°C methanol for 5 min. After removal of the methanol and washing with PBS, the cells were permeabilized with 0.5% Triton X-100 for 10 min at room temperature. The coverslips were incubated for 1 h at room temperature with the primary antibody, followed by 45-min incubation with second antibody, respectively. The stained cells were analyzed by conventional fluorescence microscopy and Zeiss (Carl Zeiss, Thornwood, NY) oil immersion lens. Color photographs were taken with a digital camera by superimposing the monochrome graphs of two channels.
Wound Healing Assay
COS-7 cells were grown on 24-well plate at 40–60% confluence and transfected with different GFP-tagged PLC-γ1 constructs. After 24–48 h, the cells reached 100% confluence, and a wound was created with a glass pipette. A nearby reference point was created by a needle. The plate was washed once and replaced with desired medium. The cells were observed under fluorescence microscope to ensure that enough cells in the leading edge of the wound were positively transfected. Both phase contrast and fluorescence images were acquired every 2 h by matching the reference point until the wound has completely closed. To calculate the rate of migration of the transfected cells, we measured the distance traveled toward the center of the wound after 8 h. We performed wound healing assays only at 8 h, which can limit the effect of the DNA synthesis. At least eight to 10 randomly chosen areas from three to four separate experiments were quantified, and an individual photograph is chosen as the example. The relative distance to the reference line is calculated and normalized to the nontransfected cells (Endo), and data are expressed as means ± SEs of the percentage of the nontransfected cells from at least eight to 10 randomly chosen areas from three to four separate experiments.
RESULTS
Colocalization of PLC-γ1 and Akt after EGF Stimulation
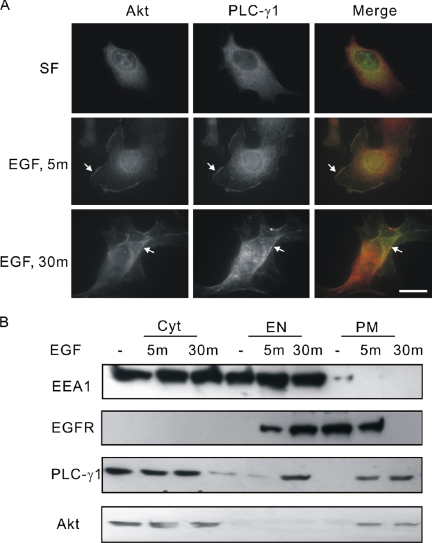
We and others have shown that PLC-γ1 translocates to the plasma membrane after EGF stimulation (Wang and Wang, 2003). It was also reported that Akt translocates to the leading edge of the plasma membrane in response to several growth factors (Carvalho et al., 2000). However, it is not clear whether PLC-γ1 and Akt colocalized in the plasma membrane after EGF stimulation. We first examine the colocalization of PLC-γ1 and Akt by indirect immunofluorescence. As shown in Figure 1A, both PLC-γ1 and Akt were cytosolic without EGF stimulation and translocated to the plasma membrane and colocalized together after EGF stimulation for 5 min. After EGF stimulation for 30 min, some PLC-γ1 was localized to the intracellular vesicles, whereas a significant portion of PLC-γ1 still colocalized with Akt in the plasma membrane (Figure 1A).
Figure 1.
Colocalization of PLC-γ1 and Akt in COS-7 cells. (A) COS-7 cells were either nontreated or treated with 100 ng/ml EGF for indicated times. The cells were then fixed and stained with anti-PLC-γ1 and anti-Akt antibodies. Colocalization (yellow) of Akt (green) and PLC-γ1 (red) is indicated by arrow. Bar, 20 μM. (B) Serum-starved COS-7 cells were either nontreated or treated with 100 ng/ml EGF for 5 or 30 min. The total cell homogenates were subcellularly fractionated into PM, EN, and Cyt. Ten percent protein of each fraction was loaded to 8% SDS-PAGE and immunoblotted with antibodies to Akt, PLC-γ1, EEA1, and EGFR.
We further studied the colocalization of PLC-γ1 and Akt by subcellular fractionation. COS-7 cells stimulated with EGF for various times were cleared of nuclei and subcellular fractionated into Cyt, PM, and EN fractions. EEA1, a protein present in both cytosol and endosomes, was used as a marker for endosome. EGFR itself is also a marker for both the plasma membrane and endosome because it stays at the plasma membrane without EGF stimulation and internalizes to endosome after EGF stimulation. As shown in Figure 1B, both PLC-γ1 and Akt were found mostly in the cytosol before EGF stimulation. After EGF stimulation for 5 min, both PLC-γ1 and Akt were detected in PM fraction. After EGF stimulation for 30 min, PLC-γ1 was detected in both PM and endosome fractions, whereas Akt was only detected in PM fraction. Together, these data indicated that PLC-γ1 and Akt were colocalized to the plasma membrane in response to EGF.
PLC-γ1 Forms an EGF-dependent Association with Akt
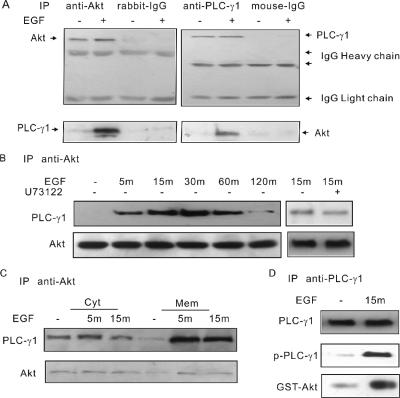
The EGF-dependent colocalization of PLC-γ1 and Akt suggests that they may actually interact each other. Coimmunoprecipitation experiments were performed to examine this possibility. The total cell lysates of COS-7 cells were first immunoprecipitated with antibody to Akt. Immunoblotting of the immunoprecipitates with anti-PLC-γ1 antibody showed that EGF stimulated strong association between Akt and PLC-γ1 (Figure 2A). Moreover, immunoprecipitation of COS-7 cell lysates with anti-PLC-γ1 antibody followed by immunoblotting with anti-Akt antibody showed EGF-dependent association between PLC-γ1 and Akt (Figure 2A). A time-course experiment was then performed to determine the timing of the interaction. As shown in Figure 2B, the association between PLC-γ1 and Akt started at 5 min and reached the highest point at 15–30 min and remained at a high level for up to 60 min after initial stimulation. After a prolonged stimulation of EGF for 120 min, the association was dropped to the background level. Inhibition of PLC-γ1 activity by U73122 did not significantly affect the association between PLC-γ1 and Akt (Figure 2B).
Figure 2.
The EGF-induced association of PLC-γ1 and Akt. (A) COS-7 cells were either not treated or treated with 100 ng/ml EGF for 30 min. The cell lysates were immunoprecipitated with either anti-Akt or anti-PLC-γ1 antibodies and then blotted with anti-PLC-γ1 and anti-Akt antibodies. In controls, the primary antibodies were replaced by normal rabbit IgG and mouse IgG. (B) Serum-starved COS-7 cells were stimulated with 100 ng/ml EGF for 5, 15, 30, 60, or 120 min. Alternatively, serum-starved COS-7 cells were pretreated by 10 μM U73122 for 10 min and then treated with 100 ng/ml EGF for 15 min. The cell lysates were then immunoprecipitated with anti-Akt antibodies, followed by blotting with anti-PLC-γ1 antibody. (C) Serum-starved COS-7 cells were either nontreated or treated with 100 ng/ml EGF for 5 or 30 min. The total cell homogenates were subcellularly fractionated into Mem and Cyt. The proteins of each fraction were immunoprecipitated with antibody to Akt, and the immunoprecipitates were immunoblotted with anti-PLC-γ1 and anti-Akt antibodies. (D) Serum-starved COS-7 cells were either nontreated or treated with 100 ng/ml EGF for 15 min, and the cell lysates were then immunoprecipitated with anti-PLC-γ1 antibodies followed by goat anti-mouse IgG conjugated with agarose. After washing three times, GST-Akt was added and incubated for 30 min. The final precipitates were washed and blotted with GST antibody.
To determine the location of the interaction, we further performed the immunoprecipitation experiments after subcellular fractionation. EGF-stimulated COS-7 cells were cleared of nuclei and subcellularly fractionated into Cyt and Mem fractions. The lysates were immunoprecipitated with antibody to Akt. Immunoblotting of the immunoprecipitates with anti-PLC-γ1 antibody showed that EGF stimulated strong association between Akt and PLC-γ1, mostly in membrane (Figure 2C). We also observed basal interaction between PLC-γ1 and Akt in cytosol, which is independent of EGF stimulation (Figure 2C).
To further examine the association between PLC-γ1 and Akt, we immunoprecipitated PLC-γ1 from COS-7 cells with or without EGF stimulation. As expected, PLC-γ1 is not tyrosine phosphorylated in the absence of EGF, but it is strongly tyrosine phosphorylated after EGF stimulation. We then incubate the immunoprecipitates with purified GST-Akt. We showed that only phosphorylated PLC-γ1 bound to GST-Akt (Figure 2D).
SH3 Domain of PLC-γ1 Is Required for the Association with Akt
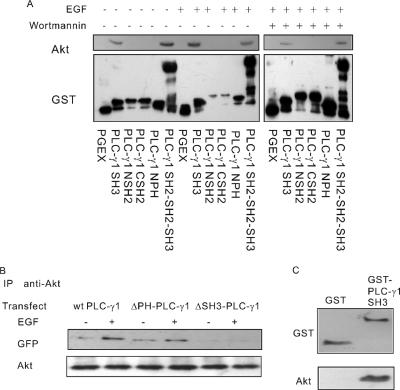
To determine which domain of PLC-γ1 is involved in the association, the binding affinity of each PLC-γ1 domain with Akt was determined in vitro using a GST pull-down method. As shown in Figure 3A, PLC-γ1 SH3 domain and a region including the SH3 and both SH2 domains (PLC-γ1 SH2-SH2-SH3) were able to bind Akt. PLC-γ1 PH domain, NSH2 domain, and CSH2 domain, in contrast, did not bind to the Akt. Interestingly, this binding was constitutive and not EGF dependent. Even treatment with wortmannin, an inhibitor of PI3K, did not abolish the association.
Figure 3.
SH3 domain of PLC-γ1 is required for the association. (A) The association of Akt with various GST fusion PLC-γ1 domains by GST pull-down. COS-7 cells were not treated, treated with 100 ng/ml EGF for 30 min, or pretreated with 100 nM wortmannin for 30 min followed by 100 ng/ml EGF for 30 min. The cell lysates were incubated with various GST fusion PLC-γ1 proteins bound to glutathione-agarose beads. Bound proteins were analyzed by immunoblotting with anti-Akt antibody. Immunoblotting with anti-GST antibody was used as loading controls. (B) COS-7 cells were transfected with wild type, ΔPH-PLC-γ1, and ΔSH3-PLC-γ1. The cells were either not treated or treated with 100 ng/ml EGF for 30 min. The cell lysates were subjected to immunoprecipitation with monoclonal anti-Akt antibody, and the resulting immunoprecipitates were subjected to immunoblotting with a polyclonal anti-GFP antibody, which reacts to both GFP and YFP. (C) In vitro binding assay. GST and GST-PLC-γ1SH3 beads were incubated with purified Akt for 30 min. After washing three times, the final precipitates were blotted with antibodies to GST and Akt.
To further determine the role of PLC-γ1 SH3 domain in mediating the interaction between PLC-γ1 and Akt, COS-7 cells were transfected with a mutant PLC-γ1 with the deletion of SH3 domain (ΔSH3-PLC-γ1). We showed by immunoprecipitation that the deletion of SH3 domain completely blocked the association between PLC-γ1 and Akt (Figure 3B). As a control, deletion of PH domain (ΔPH-PLC-γ1) had no effect on EGF-induced association between PLC-γ1 and Akt. These results indicate that SH3 domain of PLC-γ1 is required for the association between PLC-γ1 and Akt.
To further determine whether the binding between PLC-γ1 and Akt is direct, we performed in vitro binding assay by using purified Akt and GST-PLC-γ1SH3 domain. We showed that GST-PLC-γ1SH3 domain directly bound to Akt in vitro; however, GST itself did not bind to Akt. These data indicated that PLC-γ1 directly binds to Akt in vitro (Figure 3C).
Proline-rich Motifs of Akt Are Required for the Association
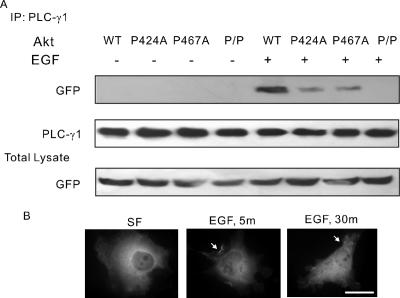
Because SH3 domain binds to the PXXP motif and Akt contains two PXXP motifs (424PFKP427 and 467PHFP470) within its C terminus, we next determined the PLC-γ1 binding sites of Akt. Akt-P424A (P424A), Akt-P467A (P467A), and Akt-P424A/P467A (P/P) were created by mutating prolines to alanines. COS-7 cells were transfected with wild-type and mutant Akt constructs. The expression level of each construct was normalized to wild type (Figure 4A). Immunoprecipitation assay showed that mutation of either P424 or P467 significantly reduced but did not abolish the Akt interaction with PLC-γ1, and mutations of both P424 and P467 fully blocked the interaction, indicating that both proline-rich motifs are involved in binding to the SH3 domain of PLC-γ1 (Figure 4A). Interestingly, similar to wild-type Akt, the mutant P/P translocated from cytosol to the plasma membrane in response to EGF (Figure 4B).
Figure 4.
Both P424 and P467 of Akt are required for the association of PLC-γ1 and Akt. (A) COS-7 cells were transfected with GFP-Akt or GFP-tagged Akt mutants P424A, P467A, and P/P. The cells were either not treated or treated with 100 ng/ml EGF for 30 min. After treatment, the cell lysates were immunoprecipitated with anti-PLC-γ1 antibody, and the resulting immunoprecipitates were immunoblotted with a polyclonal anti-GFP antibody. The PLC-γ1 blot served as the loading control, and the GFP blot of total lysate served as the control of expression. (B) COS-7 cells were transfected with GFP-Akt-P/P. The cells were then fixed and stained with anti-Akt antibody. The localization of Akt was indicated by arrow. Bar, 20 μM.
Phosphorylation of Y783 Is Essential for EGF-mediated Association between PLC-γ1 and Akt
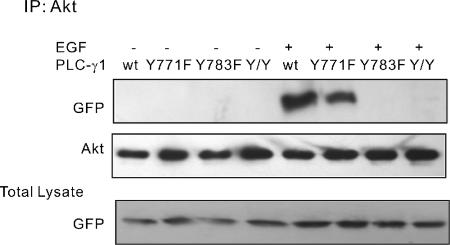
Our immunoprecipitation experiments suggest that PLC-γ1 can form a complex with Akt through its SH3 domain in an EGF-dependent manner, whereas our GST pull-down experiment suggests that the association between PLC-γ1 SH3 domain and Akt is constitutive and not dependent on EGF. This discrepancy of SH3 domain regulation between in vivo and in vitro experiments has been documented previously (Kim et al., 2000), but the mechanism is unclear. We propose that in vivo the full length PLC-γ1 adopted a conformation that restricts the interaction of its SH3 domain with Akt. EGF induced the tyrosine phosphorylation of PLC-γ1 that releases the restriction of SH3 domain. Indeed, the two major phosphorylated tyrosine residues of PLC-γ1, Y771 and Y783 reside between the PLC-γ1 C-SH2 and SH3 domain. To test our hypothesis, we generated three mutants: a mutant with the mutation of its Y771 to phenylalanine (Y771F), a mutant with the mutation of its Y783 to phenylalanine (Y783F) and a PLC-γ1 double mutant with the mutation of its both Y771 and Y783 to the phenylalanines (Y/Y). All the mutants were tagged with YFP at C terminus. COS-7 cells were transfected with YFP-tagged Y771F, Y783F, Y/Y, or wild-type PLC-γ1. Cells were stimulated with EGF, and the cell lysates were immunoprecipitated with antibody to Akt. Immunoblotting with antibodies to Akt and GFP showed that Y783F and Y/Y did not bind to Akt, whereas Y771F and wild-type PLC-γ1 bind to Akt (Figure 5). These results suggest that phosphorylation of PLC-γ1 Y783 is essential for EGF-induced association of PLC-γ1 and Akt.
Figure 5.
Regulation of EGF-induced PLC-γ1 and Akt association by PLC-γ1 Y771 and Y783. COS-7 cells were transfected with YFP-tagged wild-type PLC-γ1 or mutant PLC-γ1 Y771F, Y783F, and Y/Y. The cells were either not treated or treated with 100 ng/ml EGF for 30 min. The cell lysates were then immunoprecipitated with anti-Akt antibody. The resulting immunoprecipitates were subjected to immunoblotting with a polyclonal anti-GFP antibody. The PLC-γ1 blot served as the loading control, and the GFP blot of total lysate served as the control to show the level of expression.
AKT Phosphorylates PLC-γ1 S1248
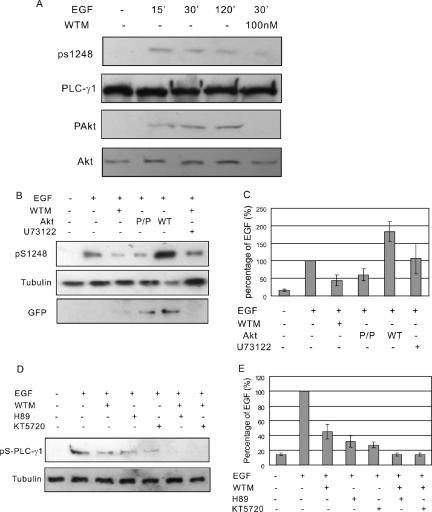
It was shown previously that PLC-γ1 undergoes serine phosphorylation after RTK stimulation (Kim et al., 1989; Rhee et al., 1990). PLC-γ1 S1248 has been identified as the major serine that is phosphorylated by RTK through PKA (Park et al., 1992), whereas other unidentified PLC-γ1 serine could be phosphorylated (Park et al., 1992). Interestingly, Akt is a serine/threonine kinase, and the minimal substrate consensus sequence for Akt is RXRXXS/T. The amino acid sequences leading to S1248 is RAREGS1248 that just confers to the minimal substrate consensus sequence for Akt. To determine whether PLC-γ1 is a substrate of Akt, we first inhibited Akt activation by treating COS-7 cells with wortmannin. Immunoblotting with antibody to phosphore Akt (pAkt) showed that wortmannin treatment complete blocked EGF-induced Akt phosphorylation. Immunoblotting with antibody to phosphore PLC-γ1 S1248 (pS1248) showed that wortmannin treatment significantly reduced the phosphorylation of PLC-γ1 S1248 (Figure 6A). To further determine the role of Akt in PLC-γ1 serine phosphorylation, we transfected COS-7 cells with wild-type Akt or mutant Akt P/P that does not bind to PLC-γ1. Immunoblotting with anti-pS1248 antibody showed that the phosphorylation of PLC-γ1 S1248 was increased by wild-type Akt but decreased by mutant Akt P/P (Figure 6, B and C). These results suggest that EGF-stimulated PLC-γ1 S1248 phosphorylation is at least partially dependent on binding to Akt. We also showed that inhibition of PLC-γ1 activity by U73122 had no effect of EGF-induced PLC-γ1 S1248 phosphorylation (Figure 6, B and C).
Figure 6.
AKT mediates EGF-induced serine phosphorylation of PLC-γ1. (A) COS-7 cells were treated with 100 ng/ml EGF for indicated times with or without 100 nM wortmannin. The total cell lysates were immunoblotted with antibodies to Akt, pAkt, PLC-γ1, and pS1248 of PLC-γ1. (B) COS-7 cells without transfection were treated with 100 ng/ml EGF or EGF and 100 nM wortmannin or U73122. COS-7 cells transfected with GFP-Akt or GFP-tagged mutant Akt P/P were either not treated or treated with EGF. Then, the cell lysates were immunoblotted with anti-pS1248 antibody. Immunoblotting with anti-tubulin antibody was used as loading control and immunoblotting with anti-GFP antibody was used to show the expression level of transfected Akt. (C) Quantification of the data from three independent experiments as described in B. The PLC-γ1 S1248 phosphorylation levels were expressed as the percentage of the level after EGF stimulation. (D) COS-7 cells were treated with 100 ng/ml EGF or EGF with PKA inhibitors H89/KT5270 or with both wortmannin and H89/KT5270. Then, the cell lysates were immunoblotted with anti-pS1248 antibody. (E) Quantification of the data from three independent experiments as described in D. The PLC-γ1 S1248 phosphorylation levels were expressed as the percentage of the level after EGF stimulation.
Because it has been suggested that PKA could phosphorylate PLC-γ1 S1248 (Park et al., 1992), we further examined the relative contribution of Akt and PKA on EGF-induced PLC-γ1 S1248 phosphorylation. We showed that inhibition of PKA by H89 and KT5270 also partially inhibited EGF-induced phosphorylation of PLC-γ1 S1248, whereas inhibition of PKA and Akt simultaneously by treating the cells with wortmannin together with H89 or KT5270 resulted in the complete inhibition of EGF-induced phosphorylation of PLC-γ1 S1248 (Figure 6, D and E).
Regulation of EGF-induced Cell Motility by Akt–PLC-γ1 Interaction
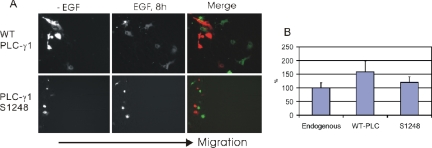
PLC-γ1 plays an essential role in EGF-dependent cell motility (Chen et al., 1994). Akt was also shown recently to regulate cell motility by an unidentified mechanism (Firtel and Chung, 2000). Because we showed that Akt binds to and serine phosphorylates PLC-γ1, we proposed that the Akt–PLC-γ1 interaction is important in mediating EGF-induced cell motility. Wound healing experiments were used to determine the cell motility. To test our hypothesis, we first determined whether PLC-γ1 S1248 phosphorylation is important in EGF-induced cell motility. We transfected COS-7 cells with either wild-type PLC-γ1 or PLC-γ1 S1248A. Wound healing assay showed that overexpression of wild-type PLC-γ1 significantly enhanced EGF-induced cell motility. However, mutation of PLC-γ1 S1248 to alanine largely abolished this enhancement (Figure 7, A and B). These results suggest that PLC-γ1 S1248 phosphorylation accounts for a significant part of cell motility induced by PLC-γ1. It is interesting to note that overexpression of PLC-γ1 S1248 slightly increased cell motility compared with nontransfected cells. This suggests that the role of PLC-γ1 in cell motility is not solely dependent on its S1248 phosphorylation.
Figure 7.
Effect of PLC-γ1 S1248 phosphorylation on EGF-induced cell motility. (A) COS-7 cells were transfected with either YFP-tagged wild-type PLC-γ1 or mutant PLC-γ1 S1248A. Confluent monolayers of serum-starved cells were not treated or treated with 100 ng/ml EGF as indicated. Cell motility was examined by wound healing assay as described in Materials and Methods. In the merged image, red shows the cells at 0 h, and green shows the cells at 8 h. All of cells migrated from the left to right as indicated. (B) Quantitation of the data from A. The data were quantitated as described in Materials and Methods.
We then examined whether overexpression of wild-type Akt in COS-7 cells increase the cell motility and whether the effects are dependent on its interaction with PLC-γ1 and dependent on PLC-γ1 activity. When COS-7 cells were transfected with GFP-Akt, wound healing assay showed that overexpression of GFP-Akt increased cell motility by 40% (Figure 8, A and B). However, further treatment with U73122 to block PLC-γ1 activity abolished this increase. This suggests that the enhancement of Akt on EGF-induced cell motility is dependent on PLC-γ1 activity. Moreover, transfection of COS-7 cells with mutant Akt P/P that does not bind to PLC-γ1 did not increase the cell motility. This suggests that the enhancement of Akt on EGF-induced cell motility is dependent on its interaction with PLC-γ1. Together, these results suggest that Akt–PLC-γ1 interaction plays an important role in EGF-induced cell motility.
Figure 8.
Effects of PLC-γ1–Akt interaction and PLC-γ1 activity on EGF-induced cell motility. (A) COS-7 cells were transfected with wild-type GFP-Akt or mutant Akt P/P. Confluent monolayers of serum-starved cells were not treated or treated with 10 μM U73122 and/or 100 ng/ml EGF as indicated. Cell motility was examined by wound healing assay as described in Materials and Methods. In the merged image, red shows the cells at 0 h, and the green shows the cells at 8 h. All of cells migrated from the left to right as indicated. (B) Quantitation of the data from A. The data were quantitated as described in Materials and Methods.
DISCUSSION
In this study, we identified a novel interaction between PLC-γ1 and Akt. We showed by immunofluorescence and subcellular fractionation that PLC-γ1 and Akt were colocalized to the plasma membrane after EGF stimulation (Figure 1). We also showed by immunoprecipitation, GST-fusion protein pull-down experiments, and in vitro binding experiments that PLC-γ1 and Akt directly bind to each other (Figures 2 and 3). It is well established that activated EGFR directly stimulates PLC-γ1 by phosphorylating its tyrosine residues and indirectly stimulates Akt by activating PI3K. Both PLC-γ1 and PI3K–Akt pathways play significant roles in EGFR-mediated cell signaling. Although both PLC-γ1 and PI3K-Akt play distinct role in cell signaling, the two pathways have been shown to interact each other. For example, PLC-γ1 and PI3K are both involved in lipid metabolism and use the same substrate (PIP2) (Carpenter and Ji, 1999). Both PLC-γ1 and PI3K–Akt pathways are involved in cell mitogenesis and motility (Chen et al., 1994). It is known that PLC-γ1 may regulate Ras activity through the activation of rasGRP, and activated Ras then stimulates the activation of PI3K (Rodriguez-Viciana et al., 1994; Ehrhardt et al., 2004). We recently showed that PI3K might affect the translocation of PLC-γ1 by generating phosphatidylinositol 3-phosphate (Wang and Wang, 2003). So far, the data regarding the interaction between PLC-γ1 pathway and PI3K–Akt pathway are exclusively between PLC-γ1 and PI3K. Here, we demonstrate that PLC-γ1 and Akt directly bind each other, which provides another layer of interaction between PLC-γ1 pathway and PI3K–Akt pathway.
We further showed that the interaction between PLC-γ1 and Akt are mediated by PLC-γ1 SH3 domain (Figure 3) and the proline-rich motifs of Akt (Figure 4). PLC-γ1 is a lipase with multiple domains, which allows the interaction of PLC-γ1 with various proteins to form large protein complexes. It is clear that PLC-γ1 SH2 domains interact with the phosphotyrosine of RTKs. However, the functions of PLC-γ1 SH3 and PH domains are still not clear. Interestingly, most newly identified interactions between PLC-γ1 and other proteins are mediated by its SH3 domains. It was recently reported that PLC-γ1 directly interact with various cellular proteins, including PLD2 (Jang et al., 2003), PIKE (Ye et al., 2002), dynamin (Choi et al., 2004), Emt (Perez-Villar and Kanner, 1999), and SOS (Kim et al., 2000) through its SH3 domain. Our studies demonstrate that Akt can be added to this list.
Akt contains two proline-rich motifs (424PFKP427 and 467PHFP470) within its C terminus. We showed that point mutation of either P424 or P467 to alanine significantly reduced but not abolish the interaction between PLC-γ1 and Akt, whereas a mutant Akt with both proline mutated to alanine (P/P) did not bind to PLC-γ1 at all (Figure 4). This suggests that both proline motifs of Akt are involved in the interaction with PLC-γ1.
Different from the interaction between SH2 domain and phosphotyrosine motif, the interaction between SH3 domain and proline-rich motifs does not require the posttranslational modification of either SH3 domain or the proline motifs. Indeed, we showed here by in vitro binding assay that the interaction between PLC-γ1 SH3 domain and Akt is constitutive (Figure 3). However, these data seem at odds with our in vivo data showing that the interaction between the full-length PLC-γ1 and Akt is dependent on EGF stimulation. Similar observation has been made for the interactions between PLC-γ1 and other proteins, such as PLD2 (Jang et al., 2003), PIKE (Ye et al., 2002), dynamin (Choi et al., 2004), and Emt (Perez-Villar and Kanner, 1999).
In this study, we provide a novel model to address this discrepancy. We proposed that in vivo, the full-length PLC-γ1 adopted a conformation that restricts the interaction of its SH3 domain with Akt. Phosphorylation of PLC-γ1 Y771 and/or Y783 by EGF stimulation releases the restriction on SH3 domain (Figure 9). Our model is supported by the following evidence. First, Y771 and Y783 are the major tyrosine residues phosphorylated by EGFR and located between PLC-γ1 SH3 and CSH2 domains. Thus, it is highly possible that the phosphorylation of Y771 and/or Y783 may have major effects on the conformation of PLC-γ1. Second, it was recently reported that in response to EGF, the phosphorylated Y783 forms an intramolecular interaction with the CSH2 domain in the activated PLC-γ1 (Poulin et al., 2005). Third, we showed in this study that double mutation of Y771 and Y783 or single mutation of Y783 blocked EGF-induced association between PLC-γ1 and Akt (Figure 5).
Figure 9.
Model to illustrate EGF-induced interaction between Akt and PLC-γ1. Before EGF stimulation, both PLC-γ1 and Akt are located in the cytosol. The conformation of inactive PLC-γ1 blocks the interaction between PLC-γ1 SH3 domain and the proline-rich motifs of Akt (left). After EGF stimulation, PLC-γ1 translocated to the plasma membrane, and its Y783 was phosphorylated by EGFR, which exposes its SH3 domain for interaction. Simultaneously, Akt was activated by EGFR through PI3K and translocated to the plasma membrane. At the plasma membrane, the PLC-γ1 SH3 domain and Akt proline-rich motifs bind to each other (right).
We also examined the role of PLC-γ1–Akt interaction in EGF-induced cell signaling. Akt is a serine/threonine kinase. The minimal substrate consensus sequence for Akt is RXRXXS/T. Interestingly, the amino acid sequence leading to S1248 is RAREGS, which suggests that S1248 could be phosphorylated by Akt. Indeed, we showed that the interaction between PLC-γ1 and Akt resulted in the phosphorylation of PLC-γ1 S1248 by Akt (Figure 6). Inhibition of Akt phosphorylation by wortmannin or disruption of PLC-γ1 and Akt interaction by mutation partially blocked EGF-induced phosphorylation of PLC-γ1 S1248 (Figure 6B), which suggests that EGF-induced PLC-γ1 S1248 phosphorylation is partially dependent on binding to Akt. Because it has been suggested that PKA could phosphorylate PLC-γ1 S1248 (Park et al., 1992), we further examined the relative contribution of Akt and PKA on EGF-induced PLC-γ1 S1248 phosphorylation. Our results indicated that both Akt and PKA partially contributed to EGF-induced PLC-γ1 S1248 phosphorylation (Figure 6, D and E).
Finally, we showed that the interaction between PLC-γ1 and Akt plays important role in EGF-induced cell motility. Both PLC-γ1 and Akt have been shown to regulate EGF-induced cell motility (Chen et al., 1994). We showed here that the enhancement of EGF-induced cell motility by PLC-γ1 is mostly abolished by mutation of its S1248 (Figure 7, A and B). This indicates that S1248 phosphorylation by Akt is important in mediating EGF-induced cell motility. We further showed that the enhancement of cell motility by Akt is abolished by blocking the interaction between Akt and PLC-γ1 and by inhibiting PLC-γ1 activity (Figure 8, A and B). All these data support an important role for Akt–PLC-γ1 interaction in EGF-induced cell motility. It was reported that inhibitors of PKC, which inhibit serine phosphorylation of PLC-γ1, also inhibit the tyrosine phosphorylation and catalytic activity PLC-γ1 (Yamada et al., 1992). Wortmannin, an inhibitor of PI3K, which inhibits Akt indirectly, also inhibits the catalytic activity of PLC-γ1. PLC-γ1 catalytic activity is involved in the EGF-mediated cell motility (Chen et al., 1994). Therefore, Akt may regulate the EGF-mediated cell motility through regulating the catalytic activity of PLC-γ1.
Whether the interaction between PLC-γ1 and Akt will also modulate Akt activity and function is still not clear. It was reported that PLC-γ2, a homologue of PLC-γ1, is required for B cell receptor-mediated cell survival (Bell et al., 2004). Akt activation is abolished by the PLC-γ1 inhibitor U73122, which suggests a possible role of PLC-γ1 in EGF-induced cell survival through the interaction with Akt (Deb et al., 2004). Therefore, whether the interaction between PLC-γ1 and Akt regulates cell survival warrants future research.
In summary, we identified a novel interaction between PLC-γ1 and Akt. We demonstrated that the interaction is mediated by the binding of PLC-γ1 SH3 domain to Akt proline-rich motifs. Both Akt proline-rich motifs interact with PLC-γ1 SH3 domain. We also provide a novel model to depict how the interaction between PLC-γ1 SH3 domain and Akt proline motifs is dependent on EGF stimulation (Figure 9). In this model, the SH3 domain of inactive PLC-γ1 is not accessible to the proline motifs of Akt. Phosphorylation of Y783 by EGFR changed PLC-γ1 conformation by forming an intramolecular association between Y783 and CSH2 domain in PLC-γ1. This conformation change results in the interaction between PLC-γ1 SH3 domain and Akt proline motifs. Furthermore, we showed that the interaction between PLC-γ1 and Akt caused phosphorylation of PLC-γ1 S1248 and plays important role in EGF-stimulated cell motility.
Acknowledgments
We thank Dr. Julian Downward for providing GFP-Akt. This work was supported in part by grants from the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and the Alberta Heritage Foundation for Medical Research (AHFMR). Z. W. is an AHFMR Senior Scholar.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–10–0918) on March 8, 2006.
References
- Alessi, D. R., Andjelkovic, M., Caudwell, B., Cron, P., Morrice, N., Cohen, P., and Hemmings, B. A. (1996). Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Anderson, D., Koch, C. A., Grey, L., Ellis, C., Moran, M. F., and Pawson, T. (1990). Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science 250, 979–982. [DOI] [PubMed] [Google Scholar]
- Andjelkovic, M., Alessi, D. R., Meier, R., Fernandez, A., Lamb, N. J., Frech, M., Cron, P., Cohen, P., Lucocq, J. M., and Hemmings, B. A. (1997). Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272, 31515–31524. [DOI] [PubMed] [Google Scholar]
- Bell, S. E., Vigorito, E., McAdam, S., Reynolds, H. M., Caraux, A., Colucci, F., and Turner, M. (2004). PLCgamma2 regulates Bcl-2 levels and is required for survival rather than differentiation of marginal zone and follicular B cells. Eur. J. Immunol. 34, 2237–2247. [DOI] [PubMed] [Google Scholar]
- Bivona, T. G., Perez, De Castro, I, Ahearn, I. M., Grana, T. M., Chiu, V. K., Lockyer, P. J., Cullen, P. J., Pellicer, A., Cox, A. D., and Philips, M. R. (2003). Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424, 694–698. [DOI] [PubMed] [Google Scholar]
- Carpenter, G., and Ji, Q. (1999). Phospholipase C-gamma as a signal-transducing element. Exp. Cell Res. 253, 15–24. [DOI] [PubMed] [Google Scholar]
- Carvalho, E., Eliasson, B., Wesslau, C., and Smith, U. (2000). Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from type II diabetic subjects. Diabetologia 43, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Chen, J., Gamou, S., Takayanagi, A., and Shimizu, N. (1994). A novel gene delivery system using EGF receptor-mediated endocytosis. FEBS Lett. 338, 167–169. [DOI] [PubMed] [Google Scholar]
- Chen, X., and Wang, Z. (2001). Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. H., et al. (2004). Phospholipase C-gamma1 is a guanine nucleotide exchange factor for dynamin-1 and enhances dynamin-1-dependent epidermal growth factor receptor endocytosis. J. Cell Sci. 117, 3785–3795. [DOI] [PubMed] [Google Scholar]
- Chou, J., Burke, N. A., Iwabu, A., Watkins, S. C., and Wells, A. (2003). Directional motility induced by epidermal growth factor requires Cdc42. Exp. Cell Res. 287, 47–56. [DOI] [PubMed] [Google Scholar]
- Datta, S. R., Dudek, H., Tao, X., Masters, S., Fu, H., Gotoh, Y., and Greenberg, M. E. (1997). Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241. [DOI] [PubMed] [Google Scholar]
- Deb, T. B., Coticchia, C. M., and Dickson, R. B. (2004). Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J. Biol. Chem. 279, 38903–38911. [DOI] [PubMed] [Google Scholar]
- del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R., and Nunez, G. (1997). Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689. [DOI] [PubMed] [Google Scholar]
- Derman, M. P., Chen, J. Y., Spokes, K. C., Songyang, Z., and Cantley, L. G. (1996). An 11-amino acid sequence from c-met initiates epithelial chemotaxis via phosphatidylinositol 3-kinase and phospholipase C. J. Biol. Chem. 271, 4251–4255. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, A., David, M. D., Ehrhardt, G. R., and Schrader, J. W. (2004). Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol. Cell Biol. 24, 6311–6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel, R. A., and Chung, C. Y. (2000). The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. Bioessays 22, 603–615. [DOI] [PubMed] [Google Scholar]
- Gual, P., Giordano, S., Williams, T. A., Rocchi, S., Van Obberghen, E., and Comoglio, P. M. (2000). Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene 19, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Hanada, M., Feng, J., and Hemmings, B. A. (2004). Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim. Biophys. Acta 1697, 3–16. [DOI] [PubMed] [Google Scholar]
- Jang, I. H., et al. (2003). The direct interaction of phospholipase C-gamma 1 with phospholipase D2 is important for epidermal growth factor signaling. J. Biol. Chem. 278, 18184–18190. [DOI] [PubMed] [Google Scholar]
- Kamat, A., and Carpenter, G. (1997). Phospholipase C-gamma1, regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 8, 109–117. [DOI] [PubMed] [Google Scholar]
- Kim, H. K., Kim, J. W., Zilberstein, A., Margolis, B., Kim, J. G., Schlessinger, J., and Rhee, S. G. (1991). PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell 65, 435–441. [DOI] [PubMed] [Google Scholar]
- Kim, M. J., Chang, J. S., Park, S. K., Hwang, J. I., Ryu, S. H., and Suh, P. G. (2000). Direct interaction of SOS1 Ras exchange protein with the SH3 domain of phospholipase C-gamma1. Biochemistry 39, 8674–8682. [DOI] [PubMed] [Google Scholar]
- Kim, U. H., Kim, J. W., and Rhee, S. G. (1989). Phosphorylation of phospholipase C-gamma by cAMP-dependent protein kinase. J. Biol. Chem. 264, 20167–20170. [PubMed] [Google Scholar]
- Kundra, V., Escobedo, J. A., Kazlauskas, A., Kim, H. K., Rhee, S. G., Williams, L. T., and Zetter, B. R. (1994). Regulation of chemotaxis by the PDGF receptor-beta. Nature 367, 474–476. [DOI] [PubMed] [Google Scholar]
- Margolis, B., Rhee, S. G., Felder, S., Mervic, M., Lyall, R., Levitzki, A., Ullrich, A., Zilberstein, A., and Schlessinger, J. (1989). EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell 57, 1101–1107. [DOI] [PubMed] [Google Scholar]
- Margolis, B., Zilberstein, A., Franks, C., Felder, S., Kremer, S., Ullrich, A., Rhee, S. G., Skorecki, K., and Schlessinger, J. (1990). Effect of phospholipase C-gamma overexpression on PDGF-induced second messengers and mitogenesis. Science 248, 607–610. [DOI] [PubMed] [Google Scholar]
- Meisenhelder, J., Suh, P. G., Rhee, S. G., and Hunter, T. (1989). Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 57, 1109–1122. [DOI] [PubMed] [Google Scholar]
- Meyer, R. D., Latz, C., and Rahimi, N. (2003). Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J. Biol. Chem. 278, 16347–16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S., Liu, X., Pawson, T., and Jove, R. (1992). Activated Src tyrosine kinase phosphorylates Tyr-457 of bovine GTPase-activating protein (GAP) in vitro and the corresponding residue of rat GAP in vivo. J. Biol. Chem. 267, 17194–17200. [PubMed] [Google Scholar]
- Patterson, R. L., van Rossum, D. B., Ford, D. L., Hurt, K. J., Bae, S. S., Suh, P. G., Kurosaki, T., Snyder, S. H., and Gill, D. L. (2002). Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell 111, 529–541. [DOI] [PubMed] [Google Scholar]
- Perez-Villar, J. J., and Kanner, S. B. (1999). Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-C gamma 1 in human T lymphocytes. J. Immunol. 163, 6435–6441. [PubMed] [Google Scholar]
- Poulin, B., Sekiya, F., and Rhee, S. G. (2005). Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc. Natl. Acad. Sci. USA 102, 4276–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, S. G., Kim, U. H., and Kim, J. W. (1990). Cross-talk between cellular signalling cascade pathways suggested by cAMP-induced phosphorylation of phospholipase C-gamma. Adv. Second Messenger Phosphoprotein Res. 24, 164–169., 164–169. [PubMed] [Google Scholar]
- Rodriguez-Viciana, P., Warne, P. H., Dhand, R., Vanhaesebroeck, B., Gout, I., Fry, M. J., Waterfield, M. D., and Downward, J. (1994). Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532. [DOI] [PubMed] [Google Scholar]
- Ronnstrand, L., Mori, S., Arridsson, A. K., Eriksson, A., Wernstedt, C., Hellman, U., Claesson-Welsh, L., and Heldin, C. H. (1992). Identification of two C-terminal autophosphorylation sites in the PDGF beta-receptor: involvement in the interaction with phospholipase C-gamma. EMBO J. 11, 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin, D., Margolis, B., Mohammadi, M., Daly, R. J., Daum, G., Li, N., Fischer, E. H., Burgess, W. H., Ullrich, A., and Schlessinger, J. (1992). SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 11, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, S. M., Coppelli, F. M., Wells, A., Gooding, W. E., Song, J., Kassis, J., Drenning, S. D., and Grandis, J. R. (2003). Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 63, 5629–5635. [PubMed] [Google Scholar]
- Wahl, M., and Carpenter, G. (1991). Selective phospholipase C activation. Bioessays 13, 107–113. [DOI] [PubMed] [Google Scholar]
- Wahl, M. I., Daniel, T. O., and Carpenter, G. (1988). Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science 241, 968–970. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and Wang, Z. (2003). Regulation of EGF-induced phospholipase C-gamma1 translocation and activation by its SH2 and PH domains. Traffic 4, 618–630. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Gluck, S., Zhang, L., and Moran, M. F. (1998). Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis. Mol. Cell Biol. 18, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and Moran, M. F. (2002). Phospholipase C-gamma 1, a phospholipase and guanine nucleotide exchange factor. Mol. Interv. 2, 352–355. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Zhang, L., Yeung, T. K., and Chen, X. (1999). Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol. Biol. Cell 10, 1621–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, A., and Grandis, J. R. (2003). Phospholipase C-gamma1 in tumor progression. Clin. Exp. Metastasis 20, 285–290. [DOI] [PubMed] [Google Scholar]
- Wells, A., Gupta, K., Chang, P., Swindle, S., Glading, A., and Shiraha, H. (1998). Epidermal growth factor receptor-mediated motility in fibroblasts. Microsc. Res. Tech. 43, 395–411. [DOI] [PubMed] [Google Scholar]
- Wells, A., Kassis, J., Solava, J., Turner, T., and Lauffenburger, D. A. (2002). Growth factor-induced cell motility in tumor invasion. Acta Oncol. 41, 124–130. [DOI] [PubMed] [Google Scholar]
- Yamada, K., Jelsema, C. L., and Beaven, M. A. (1992). Certain inhibitors of protein serine/threonine kinases also inhibit tyrosine phosphorylation of phospholipase C gamma 1 and other proteins and reveal distinct roles for tyrosine kinase(s) and PKC in stimulated, rat basophilic RBL-2H3 cells. J. Immunol. 149, 1031–1037. [PubMed] [Google Scholar]
- Ye, K., Aghdasi, B., Luo, H. R., Moriarity, J. L., Wu, F. Y., Hong, J. J., Hurt, K. J., Bae, S. S., Suh, P. G., and Snyder, S. H. (2002). Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GT-Pase PIKE. Nature 415, 541–544. [DOI] [PubMed] [Google Scholar]