Abstract
Cadherins mediate homophilic cell adhesion and contribute to tissue morphogenesis and architecture. Cadherin cell adhesion contacts are actively remodeled and impact cell movement and migration over other cells. We found that expression of a mutant cadherin-11 lacking the cytoplasmic juxtamembrane domain (JMD) diminished the turnover of α-catenin at adherens junctions as measured by fluorescence recovery after photobleaching. This resulted in markedly diminished cell intercalation into monolayers reflecting reduced cadherin-11-dependent cell motility on other cells. Furthermore, the actin cytoskeleton in cadherin-11 ΔJMD cells revealed a more extensive cortical F-actin ring that correlated with significantly higher levels of activated Rac1. Together, these data implicate the cadherin-11 cytoplasmic JMD as a regulator of α-catenin turnover at adherens junctions and actin-cytoskeletal organization that is critical for intercellular motility and rearrangement in multicellular clusters.
INTRODUCTION
Multicellularity requires directed cell rearrangements to organize tissues and to maintain tissue architecture. The cadherin transmembrane protein family mediates homotypic adhesion between cells and contributes to tissue morphogenesis. Together with the intracellular catenins and the actin cytoskeleton, they provide the molecular means to orchestrate cell rearrangements and movements of cells along other cells. (Brieher and Gumbiner, 1994; Lee and Gumbiner, 1995; Uemura et al., 1996; Niewiadomska et al., 1999).
Cadherins function at the cell surface where their ectodomains interact with like cadherin molecules on adjacent cells to physically connect cells to one another. Cadherin bonds are continuously dismantled and reformed allowing for remodeling of cell-to-cell contacts and cell rearrangements within tissues. Protein interactions at the cadherin cytoplasmic tail with either the juxtamembrane domain (JMD) or the β-catenin binding sequence (CBS) are critical for cadherin function in mediating cell-to-cell adhesion and cell rearrangements (Gumbiner, 2005). In particular, binding partners at the JMD such as p120-catenin (p120ctn), Hakai (c-Cbl-like protein), or presenilin-1 stabilize cadherin molecules at the cell surface or mediate their internalization, thereby regulating cadherin levels at the cell surface available for adhesion (Baki et al., 2001; Fujita et al., 2002; Ireton et al., 2002). Besides maintaining cadherin levels at the cell surface, p120ctn further modulates cadherin adhesive strength by signaling mechanisms that act on its amino-terminal region (Yap et al., 1998; Aono et al., 1999; Ireton et al., 2002). The distal CBS binds β-catenin (Ozawa et al., 1989; Jou et al., 1995). At the cell surface, in complex with a cadherin, the N-terminal domain of β-catenin binds α-catenin, which can interact with either β-catenin or actin filaments (Herrenknecht et al., 1991; Ozawa and Kemler, 1992). However, α-catenin does not bind β-catenin and actin filaments simultaneously (Yamada et al., 2005). Thus, α-catenin plays a critical role in the relationship of the cadherin-catenin complex and the actin cytoskeleton. When dissociated from the cadherin-β-catenin complex, α-catenin regulates actin filament assembly and mediates bundling of actin fibers (Drees et al., 2005). It also functions as a scaffold for the recruitment of proteins involved in actin cytoskeletal reorganization. Molecules that localize to adherens junctions (AJs) in an α-catenin-dependent manner include vinculin, α-actinin, and VASP (Vasioukhin et al., 2000). More recently, α-catenin has been identified as a binding partner for formin-1. In epithelial keratinocytes, formin-1 localizes to and drives actin polymerization at AJs to form actin bundles that seal the epithelial layer (Kobielak et al., 2004). Thus, formation of AJs involves recruitment of machinery for actin cytoskeletal reorganization. Importantly, actin cytoskeletal reorganization upon cadherin engagement generates the force required for changes in cell shape and/or movements of cells on other cells.
Cadherins are expressed in most epithelial and mesenchymal tissues and may influence the different nature and behavior of these respective cell types. During development, cadherin-11 expression is associated with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos (Kimura et al., 1995). Thus, cadherin-11 is considered a mesenchymal cadherin. Whereas epithelial cadherins, such as E-cadherin, are responsible for the formation and maintenance of epithelial structures, expression of cadherin-11 correlates with a migratory cellular phenotype. Evidence for the involvement of cadherin-11 in regulating cell motility has come from developmental studies, where the change from E-cadherin or N-cadherin to cadherin-11 expression correlates with cell migration and formation of new tissue structures. For example, when neural crest cells delaminate from the neural folds the neuro-epithelial cadherin, N-cadherin, is down-regulated. At the same time, cadherin-11 is expressed and may direct coordinated neural crest cell emigration (Kimura et al., 1995; Borchers et al., 2001; Locascio and Nieto, 2001). Recently, we identified cadherin-11 expression on fibroblasts in the lining layer of the synovium of diarthrodial joints (Valencia et al., 2004). In adult humans, cadherin-11 expression also has been reported on osteoblasts and endometrial stromal cells (Okazaki et al., 1994; Kimura et al., 1995; Getsios et al., 1998). Importantly, recent evidence indicates that cadherin-11 is aberrantly expressed in epithelial lineage cancer cells with a more invasive phenotype and increased risk for metastasis (Markus et al., 1999; Pishvaian et al., 1999; Tomita et al., 2000).
In this study, we explore cadherin-11 function in directing cellular rearrangements. We characterize the role of cadherin-11 and its cytoplasmic juxtamembrane domain in regulating the turnover of α-catenin at adherens junctions and actin cytoskeletal reorganization as critical determinants for cell motility, cell intercalation, and tissue extension.
MATERIALS AND METHODS
Cell Culture
The murine fibroblast cell line L-M (ATCC CCL1.3; L-cells) was grown in DMEM, high glucose, supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 10 μM nonessential amino acids (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 10% CO2. L-cell transfectants were grown in the above medium with 0.8 mg/ml hygromycin B (Invitrogen).
Plasmids and Generation of L-Cell-Cadherin-11 Stable Transfectants
Using customized double-stranded adaptors, full-length human cadherin-11 cDNA (Valencia et al., 2004) (amino acids -53 [ATG start codon] to 745) was ligated into the Hind III-BamHI sites of the expression vector pCEP4 (Invitrogen). Human cadherin-11 cDNA lacking the cytoplasmic juxtamembrane domain (cadherin-11 ΔJMD cDNA lacking amino acids 592-630) was constructed by PCR. The StuI-BamHI restriction fragment of the PCR product was inserted into the StuI-BamHI fragments of full-length cadherin-11-pCEP4. A truncated human cadherin-11 cDNA lacking the distal β-catenin binding sequence (cadherin-11 ΔCBS cDNA lacking amino acids 687-745) was also constructed by PCR (Figure 2). The PCR product encoding cadherin-11 amino acids -53 to 686 was cleaved with restriction enzymes Hind III and BamHI and inserted into the Hind III-BamHI restriction sites of the vector pCEP4. All constructs were verified by DNA sequencing. To produce cells expressing cadherin-11 at the cell surface, L-cells were transfected with pCEP4/cadherin-11-constructs or with the pCEP4 vector alone using the calcium phosphate method. Transfected cells were selected by culture in 0.8 mg/ml hygromycin B and cloned by laser-activated cell sorting.
Figure 2.
Generation of L-cells expressing cadherin-11 mutants. (A) Schematic diagram of cadherin-11 constructs used in this study. Wild-type cadherin-11 is shown on top. The cadherin-11 ΔJMD mutant has the entire juxtamembrane cytoplasmic domain deleted. The cadherin-11 ΔCBS mutant has the β-catenin-binding sequence truncated. EC1-5, extracellular cadherin repeats 1-5; TM, transmembrane domain. (B) Flow cytometric analysis of L-cell clones stably transfected with the cadherin-11 cDNA constructs. Wild-type cadherin-11 L-cells or L-cells transfected with the manipulated cadherin-11 constructs were stained with control anti-E-cadherin mAb E4.6, anti-cadherin-11 mAb 3H10, or anti-MHC class I mAb. All mAbs are mouse IgG1. (C) Biochemical analysis of L-cell transfectants. From Brij 96 lysates of L-cell transfectants (duplicates), cadherin-11 protein was immunoprecipitated using the anti-cadherin-11 mAb 3H10 and then analyzed by SDS-PAGE and Western blotting using the 5H6 anti-cadherin-11 mAb.
Antibodies and Other Reagents
The monoclonal antibodies (mAbs) cadherin-11-3H10, cadherin-11-5H6, and anti-human E-cadherin (E4.6, IgG1) were raised previously in this laboratory (Valencia et al., 2004). P3 (control mouse IgG1), affinity-purified goat anti-human IgG, and the polyclonal anti-estrogen receptor-β antibody (rabbit immunoglobulin) were from Zymed Laboratories (South San Francisco, CA); anti-p120ctn mAb (pp120, clone 98, mouse IgG1), anti-β-catenin mAb (clone 14, mouse IgG1), anti-mouse major histocompatibility complex (MHC) class I (H-2Kk; 36-7-5, mouse IgG2a), and anti-Rac1 mAb (clone 102, mouse IgG2b) were from BD Biosciences (Palo Alto, CA); and anti-α-catenin (rabbit antiserum), streptavidin-peroxidase, ribonuclease A, and trypsin inhibitor type I-S were from Sigma-Aldrich (St. Louis, MO), Alexa 488-phalloidin, Vybrant DiI cell-labeling solution, and Vybrant CFDA SE cell tracer kit were from Invitrogen, and FuGENE6 transfection reagent was from Roche Diagnostics (Indianapolis, IN). Trypsin-Tpck was purchased from Worthington Biochemicals (Lakewood, NJ), and human plasma fibronectin was from Invitrogen. Cadherin-11-Fc protein was produced and purified as described previously for E-cadherin-Fc (Higgins et al., 1998). Purified fusion protein was dialyzed into Tris-buffered saline containing 1 mM CaCl2, pH 7.4, and stored at -20°C. The purity of the fusion protein was assessed by SDS-PAGE and Coomassie blue staining, and the concentration was determined by Bradford assay using bovine serum albumin (BSA) as the standard (Bio-Rad, Hercules, CA).
Adhesion Assays
Cells were released from culture flasks using 0.02% (wt/vol) trypsin, 2 mM CaCl2, and HEPES-buffered saline (HBS) for 5 min at 37°C to minimize cadherin proteolysis and labeled with 15 μg of calcein-AM (Invitrogen). After adding 2 volumes of 0.04% soy bean trypsin inhibitor in HBS containing 1 mM CaCl2, cells were washed twice with HBS and resuspended in HBS, 0.1% BSA, 50 mM glucose, and 1 mM CaCl2. Cells were allowed to settle onto cadherin-11-Fc-coated microtiter plates for 10 min at 4°C. After incubation at 37°C for 45 or 90 min, the percentage of adherent cells was determined as described previously (Higgins et al., 1998).
Cell Migration Assay
Polycarbonate membranes (24-well insert, pore size 8 μm; Corning Glassworks, Corning, NY) were coated on both the upper and lower surface with proteins that served as migratory substrates. In particular, the membranes were incubated with fibronectin (10 μg/ml), cadherin-11-Fc fusion protein (10 μg/ml), or 1% BSA overnight at 4°C. Then, the membranes were washed twice and blocked with 1% BSA overnight at 4°C. The L-cell transfectants were serum starved overnight and released from the culture dish as described for the adhesion assay. For migration assays, 1 × 105 cells in serum-free medium were plated in the top chamber and incubated for 5 h at 37°C. Then, cells were fixed and permeabilized with ice-cold methanol for 20 min at -20°C. Cells that had not penetrated the filter were removed from top of the filter using cotton swabs, and cells that migrated to the underside of the membrane were stained with propidium iodide (1 mg/ml d-glucose in phosphate-buffered saline containing 50 μg/ml propidium iodide and 10 Kunitz units/ml ribonuclease A). Membranes were examined by fluorescence microscopy and photographed. Values for migration were expressed as the average number of migrated cells bound per microscopic field. Three microscopic fields per membrane in triplicate experiments were counted.
Immunoprecipitation
L-cell transfectants were lyzed with Triton X-100 lysis buffer, immunoprecipitated, and analyzed by Western blotting essentially as described previously (Valencia et al., 2004).
In Vitro Cadherin Induced Multicellular Organization
For cell organization experiments, L-cells either transfected with wild-type, mutant cadherin-11, or vector control were plated at 5 × 104 cells/ml (5 × 105 cells/75-cm2 surface area). Cellular organization was examined after 3-5 d of culture by phase contrast microscopy.
Immunofluorescence and Flow Cytometry
For confocal immunofluorescence microscopy, cells were fixed in 2% paraformaldehyde in HBS containing 1 mM CaCl2 for 15 min, permeabilized in 0.2% (wt/vol) saponin in 60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl, pH 6.9, for 30 min, and blocked for 30 min in 20% donkey serum. The cells were then incubated with primary antibodies at 5 μg/ml for 1 h at room temperature followed by Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa 488-conjugated phalloidin or Cy2-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Images were acquired using a 60XA/1.40 Plan Apo objective (Nikon, Tokyo, Japan) on an inverted confocal microscope (model ECLIPSE TE2000-U; Nikon) controlled by EZ-C1 version 2.20 software.
For flow cytometry, cultured cells were released essentially as described for adhesion assays and stained with primary antibodies in HBS with 1 mM CaCl2, 2% bovine serum albumin, and 2% human serum for 1 h at 4°C followed by incubation with fluorescein isothiocyanate-conjugated secondary antibody (Caltag Laboratories, Burlingame, CA) for 1 h at 4°C and analyzed on a FACScan flow cytometer (BD Biosciences).
P21-activated Kinase (PAK)-Cdc42/Rac Interactive Binding (CRIB) Pull-Down Assay
The PAK-CRIB-glutathione S-transferase (GST) fusion construct (a kind gift from H. Band, Northwestern University, IL) was expressed in DH5α Escherichia coli. L-cell transfectants were grown to subconfluence in conventional culture, starved in serum-free medium for 16 h and lysed on ice in 500 μl of lysis buffer (50 mM Tris, 150 mM NaCl2, 1% Triton X-100, 0.05% deoxycholic acid, 0.1% SDS, and protease inhibitors). Then, 35 μg of GST fusion protein bound to glutathione-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was added to each sample and incubated for 2 h. The beads were washed, and associated proteins were separated by 15% SDS-PAGE and immunoblotted with an anti-Rac1 mAb. Densitometric analysis was performed using ImageJ software (http://rsb.info.nih.gov/ij/). Bands of GTP-Rac1 were compared with those of corresponding total cellular Rac1 blots.
Cell Intercalation Assay and Time-Lapse Recording
For cell intercalation experiments, L-cell transfectants were plated onto glass-bottomed culture dishes (MatTek, Ashland, MA) and grown to confluence for 3 d and serum starved for 16 h. Before the experiment, conventionally grown L-cell transfectants were labeled with carboxyfluorecein diacetate. Then, the cells were enzymatically released essentially as described for the adhesion assay. Cells (4 × 105) were seeded on top of the monolayer of L-cell transfectants. The culture dish was placed in a temperature- and CO2-controlled incubator, and the cells were allowed to settle down onto the monolayer for 15 min. Thereafter, cells were transferred to a custom-built stage incubator on an inverted microscope (Axioskop; Carl Zeiss MicroImaging, Thornwood, NY), and an initial fluorescent image was acquired using a Spot RT charge-coupled device camera driven by IP Lab software (Scanalytics, Fairfax, VA) to identify localization of newly seeded cells. Subsequently, cell movement was documented by taking phase contrast images every 5 min for 6 h. Cell migration distance for individual cells within the monolayer was determined by manually tracing the cells. The nuclear position was recorded, and migration distance frame by frame was calculated using the Scion Image version 1.62 program (Scion, Frederick, MD). Cell intercalation of newly seeded cells into the monolayer was determined by tracking the cells frame by frame. The time required for individual cells to fully integrate into the monolayer was recorded.
Fluorescence Recovery after Photobleaching (FRAP)
L-cell transfectants were plated on coverslips, cultured for 18 h, and then transiently transfected with pEGFP-C1/αN-catenin using FuGENE6. The cells were cultured for 1 d in regular medium and starved for 16 h in serum-free medium before the experiment. Immediately before the FRAP experiments, the cells were labeled with Vybrant DiI (diluted 1:200 in serum-free medium) for 6 min at 37°C to visualize the membrane and to ensure that intimate cell-to-cell contact was preserved during the time of recording after bleaching green fluorescence. FRAP experiments were carried out using a 60XA/1.4 Plan Apo objective on a Nikon TE2000-U inverted confocal microscope with a heated stage at 37°C and EZ-C1 version 2.20 software. Fluorescence intensity was measured at low laser power before bleach, photobleached with full laser power, and recovery was followed with low laser power at 3-min intervals for 42 or 90 min. Z-sections were collected at the end of recordings to ensure that no major shift in the focal plane had occurred. Fluorescence intensity was analyzed frame by frame at intercellular junctions using ImageJ software. Curves were normalized to prebleach intensity by scaling the unbleached region of the image to that of the prebleached region. Recovery rates were calculated as a single exponential recovery using Kaleidograph software (Synergy Software, Reading, PA). Statistical analysis was performed using the Student's t test.
Online Supplemental Video 1
The cadherin-11 cytoplasmic juxtamembrane domain (JMD) regulates rearrangements of cell-to-cell contacts and cell movements and intercalation into monolayers. Dispersed, wild-type cadherin-11 L-cells were seeded over an existing monolayer of wild-type cadherin-11 L-cells (left), or dispersed ΔJMD cadherin-11 L-cells were seeded over an existing monolayer of ΔJMD cadherin-11 L-cells (right). Arrows at the beginning of the video indicate newly seeded cells, as confirmed by fluorescent labeling (not shown). One frame was captured every 5 min for 6 h, and the video play rate is six frames/s (∼1800-fold faster than real time). Dispersed wild-type cadherin-11 L-cells rapidly intercalated into the dynamic wild-type monolayer. In contrast, some ΔJMD cadherin-11 L cells failed to intercalate into the less dynamic cadherin-11 ΔJMD L-cell monolayer, even after 6 h. Failing to intercalate, these cells sometimes spread somewhat on top of the monolayer (arrows at the end of the video).
RESULTS
Cadherin-11 Mediates Cell-to-Cell Adhesion and Functions as a Migratory Substrate
To establish a model system in which cadherin-11 function could be defined, we generated L-cell clones stably expressing cell surface cadherin-11. L-cells are murine fibroblasts that are frequently used for expression of transfected cadherins because they lack expression of an endogenous cadherin, but they contain the intracellular catenins needed for proper cadherin function. Cell condensation is the most obvious morphogenic transition mediated by cadherins (Gumbiner, 1996). Indeed, upon cadherin-11 expression, cultured L-cells were connected to one another, condensed, and formed aggregates (Figure 1A). At higher cell density, the cells formed extensive and intimate contacts along their surfaces, ultimately leading to a continuous sheet of cells. In contrast, control L-cells transfected with empty vector were loosely organized and the assembly of cells into a tissuelike structure was not observed (Figure 1A).
Figure 1.
Cadherin-11 mediates cell-to-cell adhesion, cell aggregation, and induces cell migration. (A) Cadherin-11 mediates cell condensation. Vector-transfected L-cells or L-cells transfected with cadherin-11 were seeded at equal density and cultured for 4 d. L-cells expressing cadherin-11 formed clusters with extensive cell-to-cell contact, whereas vector L-cells were randomly distributed without specific cell-to-cell interactions. Bars, 20 μm. (B) Adhesion of L-cell transfectants to cadherin-11-Fc. Serial dilutions of purified cadherin-11-Fc were immobilized on microtiter plate wells coated with polyclonal goat anti-human IgG antibody and blocked with BSA. The adhesion of control L-cells (vector L-cells) or cadherin-11-transfected L-cells was determined in triplicate as described in Materials and Methods. The results are expressed as the mean percent of cells that were adherent ± 1 SD. (C) Cadherin-11 engagement induces cell migration. Boyden chambers were coated on both the upper and the lower surface with fibronectin (10 μg/ml) or cadherin-11-Fc (10 μg/ml) and blocked with BSA. Random migration was assessed as described in Materials and Methods. Values are means ± 1 SD of triplicates (n = 4).
To test the capacity of the cadherin-11 L-cells to specifically bind cadherin-11, we used the cadherin-11-Fc fusion protein in static adhesion assays (Valencia et al., 2004). Binding of L-cell transfectants to wells coated with nanogram quantities of cadherin-11-Fc could be detected in the presence of 1 mM CaCl2. At 30 ng/well, 41% of the cadherin-11 expressing L-cells adhered and maximal adhesion occurred at 90 ng/well cadherin-11-Fc with 68% of cells adherent (Figure 1B). Thus, a dose-dependent adhesion of cadherin-11 L-cells to the cadherin-11 fusion protein was apparent. In contrast, no adhesion of cadherin-11 L-cells could be detected in wells coated with 1000 ng/well human IgG1, and no binding activity to the cadherin-11 fusion protein could be detected for L-cells transfected with control vector, indicating that the observed adhesion was based on specific cadherin-11 homophilic interactions (Figure 1B).
Cadherins are instrumental in the process of multicellular organization and tissue morphogenesis. This process involves directed migration of cells and requires cellular rearrangements where cells remain in close contact while migrating over each other. These cellular rearrangements provide a means to extend tissue during development or to extend tumor projections into host tissues during tumor cell invasion. We used the cadherin-11 fusion protein to isolate the role of cadherin-11 in mediating cell movement upon cadherin-11 engagement. Boyden chamber membranes were precoated on both the upper and lower surfaces with cadherin-11 fusion protein or control protein (BSA, fibronectin), and haptokinesis of L-cells was examined by counting the cells that migrated from the upper surface through the pores to the underside of the membrane (Figure 1C). Strikingly, upon cadherin-11 engagement, the cadherin-11 expressing L-cells exhibited a strong migratory activity (483 ± 28 cells/low power field [LPF]) that was approximately 10-fold higher than the migratory activity of empty vector control L-cells (42 ± 21 cells/LPF). The basal level of cell motility of vector control L-cells plated onto cadherin-11 fusion protein coated membranes was similar to the motility of cadherin-11 L-cells on BSA (42 ± 21 and 31 ± 15, respectively), indicating that the cadherin-11-Fc fusion protein-induced migration was specific to cadherin engagement. Furthermore, the level of cadherin-11-induced motility was equivalent to fibronectin-induced haptokinesis (Figure 1C). Thus, our results show that cadherin-11 can induce cell migration to a similar extent as fibronectin in our model system.
Generation of Mutant Cadherin-11 L-Cells
Accumulating evidence indicates that cadherins exert their biological effects through interactions with intracellular molecules via their cytoplasmic domains. At the cadherin cytoplasmic tail, two distinct highly conserved domains have been defined and referred to as the JMD and the CBS. To evaluate the potential role of these domains in cell motility upon cadherin-11 engagement, we generated constructs that either lacked CBS (cadherin-11 ΔCBS) or JMD (cadherin-11 ΔJMD) of the cytoplasmic tail (Figure 2A). Plasmids encoding the various constructs were transfected into the parent L-cell clone that was also used to generate wild-type cadherin-11 expressing L-cells and vector control L-cells. Cell clones were selected on the basis of homogeneous cadherin-11 expression as determined by flow cytometry (Figure 2B). At least two independent clones (three or more for most of the experiments) were selected for all constructs to ensure that any observed effects were not due to clonal variation in the immortalized L-cells. We confirmed expression of wild-type (119-kDa) and mutant cadherin-11 (115-kDa, cadherin-11 ΔJMD; 113-kDa, cadherin-11 ΔCBS) molecules of the expected sizes by immunoprecipitation of cell lysates using an antibody against cadherin-11 (Figure 2C).
Formation of Adherens Junctions in L-cells Expressing Wild-Type or Mutant Cadherin-11
To evaluate the assembly of AJs at sites of cell-to-cell contact in L-cells transfected with the cadherin-11 constructs, immunofluorescence studies were performed. Cells were plated onto cover glasses at low density (20% confluence) in regular media. After 4 d in culture, the cells were processed for indirect immunofluorescence and stained with antibodies against cadherin-11, p120ctn, β-catenin, or α-catenin. At sites where the wild-type cadherin-11 L-cells formed intimate cell-to-cell contacts, the anti-cadherin-11 antibody labeled a mostly linear or jagged pattern of AJs. Cadherin-11 staining at the cell periphery was specifically detected at AJs, because no cadherin-11 staining was seen at sites without cell-cell contact. In wild-type cadherin-11 L-cells, we observed the same staining pattern for p120ctn, β-catenin, and α-catenin, indicating localization of p120ctn, β-catenin, and α-catenin to AJs (Figure 3). In contrast, in cadherin-11 ΔJMD L-cells, p120ctn did not localize to cell-to-cell contact sites, whereas the antibodies against cadherin-11, β-catenin, or α-catenin still labeled AJs (Figure 3). This suggests that in the ΔJMD mutant, p120ctn binding, but not β-catenin or α-catenin binding to the junctional complex was disrupted. In cadherin-11 ΔCBS L-cells, immunofluorescence revealed staining for both cadherin-11 and p120ctn at sites of cell-to-cell contact, but not for β-catenin or α-catenin. However, in these cells the cadherin-11 staining pattern was patchy compared with cells expressing wild-type cadherin-11 or cadherin-11 ΔJMD. In particular, the typical alignment of the plasma membranes of interconnected cells could not be detected (Figure 3). This staining pattern suggests that in cadherin-11 ΔCBS L-cells the organized formation of intimate cell-to-cell contacts was greatly impaired.
Figure 3.
Adherens junction formation in L-cells transfected with wild-type cadherin-11 or mutant cadherin-11. L-cells were cultured on coverslips for 4 d and processed for immunofluorescence microscopy. Confocal microscopy revealed colocalization of p120-catenin (red) and α-catenin (green) at sites of cell-to-cell contact in wild-type cadherin-11 L-cells (yellow in merged image indicates overlap of red and green labels). Extensive intercellular junctions in cadherin-11 ΔJMD L-cells were labeled with the α-catenin antiserum but not with the mAb against p120-catenin. In cadherin-11 ΔCBS L-cells disorganized intercellular junctions were labeled with the p120-catenin mAb but not the α-catenin antiserum. Bars, 10 μm.
Distinct Pattern of Actin Cytoskeletal Organization in Wild-Type Cadherin-11 L-Cells and Cadherin-11 ΔJMD L-Cells
We initially examined the L-cell clones for their long-term in vitro behavior using phase contrast microscopy. The L-cells were seeded as single cells at equal numbers and cultured for 5 d in regular media. Despite cell surface cadherin-11 expression, the cadherin-11 ΔCBS L-cells did not condense and the formation of intimate cell-to-cell contacts could not be detected; instead, their contacts seemed to be random and dependent solely on cell density, much as was the case for the vector control L-cells (Supplemental Figure 1A). Consistent with organized and extensive AJ formation (see above), the cadherin-11 ΔJMD L-cells demonstrated extensive and intimate cell-cell contacts along their surfaces. In this respect, their behavior was similar to wild-type cadherin-11 L-cells. On clustering, however, morphological differences between wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells became apparent (Figure 4A). Wild-type cadherin-11 L-cells mainly preserved their elongated, spindlelike cell shape even after formation of extensive cell-to-cell contacts. By contrast, the cadherin-11 ΔJMD L-cells demonstrated a more polygonal cell shape, most strikingly in cells with extensive cell-to-cell contacts. In addition to the difference in cell shape, we observed a distinct pattern of aggregation in the cadherin-11 ΔJMD L-cells. Although wild-type cadherin-11 L-cells clustered into aggregates that reached out and fused with neighboring aggregates to establish a continuous network of cells, cadherin-11 ΔJMD L-cells clustered into islands that were mostly isolated and did not connect to surrounding clusters (Figure 4A).
Figure 4.
Distinct multicellular organization and pattern of actin cytoskeletal organization in wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells. (A) In vitro behavior of wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells. Equal numbers of L-cells were plated on culture dishes in conventional media. After 5 d in culture, L-cells transfected with wild-type cadherin-11 formed aggregates with cells reaching out to surrounding aggregates to form a continuous network of cells. By contrast, cadherin-11 ΔJMD L-cells formed clusters that were isolated and seldom connected to surrounding clusters. Bars, 50 μm. (B) Cadherin-11-dependent actin cytoskeletal reorganization. L-cells were plated at intermediate density and grown to high density for 3 d. Then, L-cells were processed for fluorescence microscopy using phalloidin-Alexa 488 to label F-actin (green). In cadherin-11 ΔJMD L-cells, F-actin staining revealed thick bundles of fibers forming a cortical F-actin ring underlying the intercellular junctions. By contrast, wild-type cadherin L-cells exhibited a different pattern of cytoskeletal organization with radial F-actin fibers that reach into intercellular junctions and were mainly oriented in parallel to the longitudinal axis of the spindle-shaped cells. (C) L-cells were seeded at intermediate density and cultured overnight to allow only few cell-to-cell junctions to be formed. The cells were processed for indirect immunofluorescence using phalloidin to label F-actin (green) and anti-cadherin-11 3H10 to label AJs (red). Cadherin-11 ΔJMD L-cells demonstrated radial F-actin fibers that reached into AJs (arrowheads pointing to examples) in addition to thick F-actin bundles underlying intercellular junctions (thin arrows). By contrast, wild-type cadherin-11 L-cells exhibited radial F-actin fibers that reached into intercellular junctions (arrowheads pointing to examples), but thick bundles of cortical F-actin underlying the intercellular junctions were lacking.
The distinct cellular morphology of cells expressing wild-type or ΔJMD cadherin-11 observed in Figure 4A most likely results from differences in actin cytoskeletal reorganization upon cell-to-cell contact formation. The assembly of AJs generates signals that drive actin cytoskeletal rearrangements resulting in changes in cell shape and cell mobility (Adams et al., 1998; Vasioukhin et al., 2000). To probe cadherin-11-dependent actin cytoskeletal reorganization in wild-type cadherin-11 or cadherin-11 ΔJMD L-cells, we first plated cells at 50% confluence and allowed the cells to grow into a continuous sheet of cells. Wild-type cadherin-11 L-cells showed radial F-actin fibers as labeled with Alexa 488-phalloidin. These actin fibers radiated internally from AJs and were mainly oriented along the longitudinal cell axis (Figure 4B). In contrast, cadherin-11 ΔJMD L-cells demonstrated markedly thick bundles of cortical actin fibers that formed a cortical actin ring underlying the intercellular junctions (Figure 4B). To determine whether the distinct pattern of actin fibers was dependent on cell-to-cell contact formation, we plated L-cells at lower density and cultured them overnight allowing only a few cell-to-cell contacts to be formed. In cadherin-11 ΔJMD L-cells, radial actin fibers emanating from AJs were noted in addition to numerous cortical actin fibers underlying the intercellular junctions. These cortical fibers were only present at sites of cell-to-cell contact, as highlighted by coimmunofluorescence staining using an antibody against cadherin-11 (Figure 4C). Consistent with the observation at higher cell density, wild-type cadherin-11 L-cells demonstrated radial F-actin fibers that originated from AJs but that lacked thick bundles of cortical F-actin fibers underlying the intercellular junctions (Figure 4C, compare top with bottom panels, thin arrows identify thick actin bundles underlying cell junctions). The observed radial F-actin fibers that were associated with clusters of AJs indicate regulated cytoskeletal reorganization upon cell-to-cell contact in both the wild-type and ΔJMD cadherin-11 L-cells. A prominent cortical F-actin ring underlying the intercellular junctions, however, was only found in the cadherin-11 ΔJMD L-cells. In cadherin-11 ΔCBS L-cells, F-actin organization that was linked to cadherin-11-mediated intercellular junctions could not be detected. These cells demonstrated a pronounced actin rim at the cell periphery but lacked F-actin fibers radiating internally from AJs as well as bundles of F-actin fibers underlying the intercellular junctions (Supplemental Figure 1B). This result is consistent with the function of the cadherin cytoplasmic CBS in mediating the link to the actin cytoskeleton.
Cadherin-11 Engagement Down-Modulates Rac1 Activity
Our analyses suggest differences in the regulation of cadherin-11-dependent actin cytoskeletal reorganization in wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells. The Rho-family GTPases are central regulators of actin cytoskeletal reorganization and cell-to-cell adhesion. Importantly, recent studies indicate that E-cadherin engagement increases Rac1 activity in epithelial cells (Noren et al., 2001; Kovacs et al., 2002). Moreover, cellular levels of Rac1 activity determine cell shape and cell-to-cell adhesion dynamics in epithelial cells (Doye et al., 2002; Palacios and D'Souza-Schorey, 2003).
Because changes in actin cytoskeletal organization were most prominent in confluent cells, we monitored Rac1 activity in L-cell transfectants grown to confluence. The levels of GTP-bound Rac1 were measured using the PAK-GST pull-down assay. We observed that in vector L-cells that do not express cadherins levels of Rac1-GTP were high. Interestingly, in wild-type cadherin-11 L-cells the levels of Rac1 activity were low. This observation contrasts with findings in epithelial cells where E-cadherin engagement is associated with Rac1 activation. We further observed that the cadherin-11 cytoplasmic domains determine levels of Rac1 activity. Notably, the levels of Rac1 activity were significantly higher in the cadherin-11 ΔJMD L-cells as well as the cadherin-11 ΔCBS L-cells compared with wild-type cadherin-11 L-cells (Figure 5, A and B, and Supplemental Figure 1C). These results suggest that wild-type cadherin-11 engagement actively down-modulates Rac1-GTP, whereas ΔJMD cadherin-11 as well as ΔCBS cadherin-11 binding fails to down-modulate Rac1 activity. Together, our results demonstrate that in the context of a functional link between cadherin-11 junctions and the actin cytoskeleton, cellular Rac1-GTP levels correlate with cell shape. Thus, polygonal cell shape in the cadherin-11 ΔJMD L-cells is reflected by higher Rac1 activity, whereas spindle-shaped cell morphology upon wild-type cadherin-11 engagement is associated with lower levels of Rac1 activity.
Figure 5.

Cadherin-11 engagement down-modulates Rac1 activity. (A and B) The levels of Rac1-GTP were measured in confluent L-cells expressing wild-type cadherin-11, cadherin-11 ΔJMD, or control vector. Data are representative of four independent experiments. Densitometric analysis was performed using ImageJ software.
Deletion of the Cadherin-11 Cytoplasmic JMD Reveals Differential Regulation of Cadherin-11 Adhesive Function and Cadherin-11-induced Migration
Cell migration is critically dependent upon cellular adhesive interactions with the molecular stratum upon which cells crawl as well as actin polymerization and reorganization. Because the cadherin-11 ΔJMD L-cells were capable of forming organized AJs that were linked to the actin cytoskeleton, we further analyzed these cells by comparing their migratory response upon cadherin-11 engagement to that of the wild-type cadherin-11 L-cells. For this purpose, we used the cadherin-11-Fc fusion protein coated to both sides of the membranes of Boyden chambers. L-cells expressing empty vector or cadherin-11 ΔCBS (devoid of a functional link to the actin cytoskeleton) showed only little migratory activity (67 ± 22 cell/LPF and 132 ± 18 cells/LPF respectively) (Figure 6A and Supplemental Figure 1D). In contrast, wild-type cadherin-11 L-cells exhibited robust migratory activity from the upper to the lower surface of the membrane (494 ± 31 cells/LPF; see above). Cadherin-11 ΔJMD L-cells attached and spread upon plating on cadherin-11-Fc fusion protein in a similar way as the wild-type cadherin-11 L-cells (our unpublished data); however, they demonstrated twofold decreased migratory activity (243 ± 36 cell/LPF) (Figure 6A).
Figure 6.

The cadherin-11 cytoplasmic JMD determines cadherin-11-induced migration but not cadherin-11 adhesive function. (A) The cadherin-11 juxtamembrane domain regulates cadherin-11-induced migration. Values are mean ± 1 SD of triplicates (n = 3). Cadherin-11-induced migration assays were performed as described in Materials and Methods. (B) Specific adhesion to cadherin-11-Fc was similar for cadherin-11 ΔJMD L-cells and wild-type cadherin-11 L-cells. The adhesion assay was conducted as described in Materials and Methods using vector control L-cells, wild-type cadherin-11 L-cells, or L-cells expressing the cadherin-11 ΔJMD mutant and microtiter plates directly coated with the indicated concentrations of cadherin-11-Fc protein. The results are expressed as the mean percentage of cells that were adherent ± 1 SD (n = 4).
To address the question whether the decreased migratory response upon cadherin engagement in the cadherin-11 ΔJMD L-cells is associated with alterations in adhesive activity, we determined their adhesive capacity in comparison with the wild-type cadherin-11 L-cells using static adhesion assays. The cadherin-11 ΔJMD L-cells demonstrated dose-dependent cadherin-11-specific binding after 90-min incubation that was similar to the wild-type cadherin L-cells (Figure 6B). In comparison, the adhesive capacity of cadherin-11 ΔCBS L-cells was diminished by 50% (Supplemental Figure 1E). Thus, in cadherin-11 ΔCBS L-cells impaired migration was associated with decreased adhesive activity, consistent with the notion that a functional link between AJs and the actin cytoskeleton is required for both cadherin adhesive function and cadherin-mediated intercellular motility. In cadherin-11 ΔJMD L-cells, however, cadherin-11 engagement resulted in stable adhesive interactions similarly to wild-type cadherin-11 L-cells, whereas their migratory response was twofold diminished.
Cadherin-11 Promotes Cell Motility within Monolayers That Is Regulated by the Cytoplasmic JMD
The striking difference between wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells in the migratory response upon cadherin-11 binding in Boyden chamber assays suggests a role for the cadherin-11 cytoplasmic JMD in regulating intercellular motility. Therefore, we examined the motility of individual cells within monolayers of wild-type cadherin L-cells or cadherin-11 ΔJMD L-cells. Cells were plated at equal density and grown to confluence allowing intimate cell-to-cell contacts to be formed. For wild-type cadherin-11 L-cells, time-lapse videomicroscopy revealed spindlelike cell shapes and movement of cells within the monolayer with cells constantly remodeling their cellular contacts. The cells migrated in random patterns, frequently changing the direction of movement (Supplemental Video 1). By contrast, the monolayer formed by the cadherin-11 ΔJMD L-cells covered the surface area more evenly with individual cells exhibiting polygonal cell shape and more intimate cell-to-cell contact around the entire cell body. These cells exhibited more static cell-to-cell contacts and the migratory activity within the monolayer was low (Supplemental Video 1). Tracking of individual cells for 6 h revealed a 50% decrease in the mean cumulative migration distance in cadherin-11 ΔJMD L-cells (8.7 ± 0.7 μm) compared with wild-type cadherin-11 L-cells (17.2 ± 0.9 μm) (Figure 7). The differences between the wild-type and ΔJMD cadherin-11 L-cells observed are consistent with the results obtained from Boyden chamber experiments and further suggest that cadherin-11 promotes movement of cells along other cells that is controlled by its cytoplasmic JMD.
Figure 7.
Cadherin-11 promotes intercellular motility that is controlled by the cytoplasmic JMD. To determine dynamic rearrangements of cell-to-cell contacts within monolayers of wild-type cadherin-11 L-cells or cadherin-11 ΔJMD L-cells, we performed time-lapse experiments as described in Materials and Methods. Tracking of individual cells for 6 h using Scion Image version 1.62 software revealed marked differences in the cumulative migration distance in cells within the monolayer formed by wild-type cadherin-11 L-cells compared with cadherin-11 ΔJMD L-cells. Each line represents an individual cell. Thirty cells for each cell type were analyzed. The bar graph represents mean ± SEM of the cells analyzed.
Cadherin-11 Promotes Cell Intercalation
Radial tissue extension is achieved by cell intercalation where cells move along other cells and continuously reorganize their cell-to-cell junctions. To examine in vitro intercalation in wild-type and ΔJMD cadherin-11 L-cells, we plated enzymatically dispersed and fluorescently labeled cells on top of unlabeled confluent L-cells expressing either wild-type or ΔJMD cadherin-11. Tracking of individual cells using time-lapse videomicroscopy revealed that wild-type cadherin-11 L-cells placed on top of a monolayer efficiently probe the cells beneath them. Short cellular processes from the upper cells reach out to cells forming the monolayer. Most of the wild-type cadherin-11 L-cells quickly intercalated when plated on top of a monolayer formed by wild-type cadherin-11 L-cells (Figure 8A and Supplemental Video 1). In fact, 2 h after plating, 50% of the cells were already integrated into the monolayer (Figure 8B). Cadherin-11 ΔJMD L-cells that were plated on top of cadherin-11 ΔJMD L-cells explored the cells beneath in a similar way as the wild-type cadherin-11 L-cells, but their capacity to intercalate was dramatically reduced. Intercalation of 50% of the cells plated on top of the monolayer took more than twice as long compared with wild-type cadherin-11 L-cells plated on top of wild-type cadherin-11 L-cells (Figure 8, A and B, and Supplemental Video 1). When wild-type cadherin-11 L-cells were plated on top of cadherin-11 ΔJMD L-cells, their capacity to intercalate into the monolayer was substantially decreased (50% cell intercalation after 4 h; our unpublished data). These experiments indicate that cell intercalation is significantly dependent upon the cadherin-11 ΔJMD L-cells forming the monolayer. Together, these results reveal that in wild-type cadherin-11 L-cells intercellular contacts are highly dynamic and implicate the cadherin-11 cytoplasmic JMD in the regulation of cellular rearrangements.
Figure 8.
Cadherin-11 promotes cell intercalation. (A) Sequence of pictures, selected from individual time-lapse videomicroscopy experiments, showing intercalation of a wild-type cadherin-11 L-cell into a monolayer formed by wild-type cadherin-11 L-cells (left) and failure of intercalation of cadherin-11 ΔJMD L-cells into a monolayer formed by cadherin-11 ΔJMD L-cells (right). Bars, 10 μm. (B) Quantification of cell intercalation experiments. Cell intercalation was calculated after tracking individual cells for 6 h. One representative experiment out of four is shown.
At AJs, α-Catenin Is More Frequently Exchanged in Wild-Type Cadherin-11 L-Cells Compared with Cadherin-11 ΔJMD L-Cells
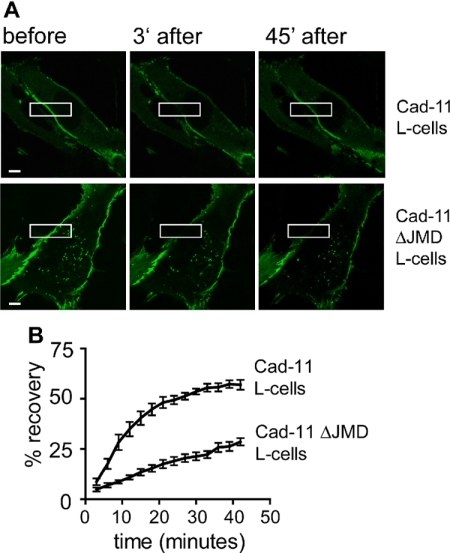
To probe the dynamic nature of a critical molecular component of AJs, we analyzed α-catenin exchange at sites of cell-to-cell contact in wild-type cadherin-11 L-cells and cadherin-11 ΔJMD L-cells using FRAP. Importantly, α-catenin mediates the functional link between the cell-to-cell adhesion complex and the actin cytoskeleton; therefore, its behavior is likely critical for actin dynamics at intercellular junctions. We transiently transfected L-cells with the DNA construct encoding GFP-αN-catenin. GFP-αN-catenin localized to sites of cell-to-cell contact in both the wild-type cadherin-11 L-cells and the cadherin-11 ΔJMD L-cells but not in cadherin-11 ΔCBS L-cells or vector control L-cells (our unpublished data). This suggested that GFP-αN-catenin recruitment to cell-to-cell contact sites was dependent on cadherin-11 and its cytoplasmic distal domain (CBS). FRAP studies revealed that the rate of fluorescence recovery at sites of cell-to-cell contact was significantly reduced in the cadherin-11 ΔJMD L-cells (mean ± SD = 0.0103 ± 0.002% recovery/min) compared with wild-type cadherin-11 L-cells (mean ± SD = 0.0681 ± 0.041% recovery/min; p < 0.01) (Figure 9, A and B). When fluorescence recovery was followed for an extended time period of 90 min, the percentage of recovery for the wild-type and ΔJMD L-cells reached 62 and 63%, respectively. Based on these experiments, the half time of recovery was 11.0 ± 3.4 min (mean ± SD) for the wild-type cadherin-11 L-cells and 42.0 ± 6.0 min (mean ± SD) for the ΔJMD cadherin-11 L-cells (calculated from maximal recovery after 90 min). The decreased rate of fluorescence recovery in cadherin-11 ΔJMD L-cells indicates diminished α-catenin turnover at AJs in these cells compared with wild-type cadherin-11 L-cells.
Figure 9.
The cadherin-11 cytoplasmic JMD promotes α-catenin turnover at AJs. (A) L-cells stably expressing wild-type cadherin-11 or cadherin-11 ΔJMD were seeded onto coverglasses, transiently transfected with GFP-αN-catenin, and grown to near confluence for 2 d. Fluorescence intensity recovery was documented on a confocal microscope after photobleaching. Bars, 5 μm. (B) Shown are the means of percentage of recovery curves ± SEM of at least seven independent experiments for each cell type.
DISCUSSION
Cadherin-11 Is a Cellular Motility Factor
Here, we show cadherin-11 is a cellular motility factor. Cadherin-11 actively directs remodeling of cell-to-cell contacts and actin cytoskeletal reorganization, which translate into movement of cells along other cells. Furthermore, we identify the cadherin-11 cytoplasmic JMD as a critical determinant of cadherin-11 driven motility by regulating both actin cytoskeletal reorganization and α-catenin turnover at AJs.
Consistent with the function of classic cadherins, cadherin-11 confers on cells the ability to form stable, homophilic interactions. However, these intercellular junctions are not static but continuously reorganize in a cadherin-11-driven manner. Immobilized cadherin-11 substrate in Boyden chamber assays induced strong migratory activity in cadherin-11-expressing cells comparable with that of fibronectin. These experiments suggest that cadherin-11 promotes movement by directing reorganization of homophilic interactions and actin cytoskeletal reorganization that elicits protrusive and traction forces required for movement. This cadherin-11 driven motility may be important in tissue spreading or extension where cells move and rearrange while maintaining intimate contacts.
The Function of the Cadherin-11 Cytoplasmic JMD in Regulating Cellular Rearrangements and Intercellular Motility
Cells that were transfected with a deletion mutant that lacked the cytoplasmic JMD demonstrated decreased intercellular motility. Because these cells were capable of forming stable adhesive interactions similar to wild-type cadherin-11 L-cells, we hypothesized that their impaired motility was due to less dynamic remodeling of cell-to-cell contacts. Indeed, integration of these cells into a monolayer formed by the same cells was profoundly diminished compared with wild-type cadherin-11-expressing L-cells. Furthermore, fluorescence recovery after photobleaching studies revealed reduced α-catenin turnover at AJs in comparison with the wild-type cadherin-11 L-cells. In addition to altered remodeling of cell-to-cell contacts in the cadherin-11 ΔJMD L-cells, we observed a striking difference in actin cytoskeletal organization. Similar to epithelial cells, cadherin-11 ΔJMD L-cells revealed a prominent cortical F-actin ring underlying the intercellular junctions. By contrast, wild-type cadherin-11 L-cells exhibited actin fibers that radiated internally from intercellular junctions along the longitudinal axis of the spindlelike cells. These observations implicate the cadherin-11 cytoplasmic JMD in the regulation of the actin cytoskeleton and α-catenin turnover at AJs that influence cellular rearrangements and cadherin-11-driven intercellular motility.
Among cadherin domains, the cytoplasmic JMD is highly conserved in its core region, which has been identified as the binding site of p120ctn (Ozawa and Kemler, 1998; Yap et al., 1998). Dependent on the cell type, distinct isoforms of p120ctn or phosphorylation at its regulatory amino-terminal end can alter the strength of the cadherin adhesive bonds (Yap et al., 1998; Aono et al., 1999; Thoreson et al., 2000). The cytoplasmic JMD, however, has also been found to regulate cadherin adhesive function independently of p120ctn binding (Ozawa, 2003). In our studies, we find that expression of cadherin-11 ΔJMD in fibroblasts yielded adhesive activity similar to wild-type cadherin-11, indicating that deletion of the cytoplasmic JMD did not alter cadherin-11 adhesive function. However, the striking intercellular motility of wild-type cadherin-11 L-cells was lost.
Several features might account for the JMD-controlled mechanism that allows cellular rearrangements and intercellular motility in wild-type cadherin-11-expressing cells. First, cadherin-11 engagement may initiate signaling events at the cytoplasmic JMD that impact actin cytoskeletal reorganization and the stability of the junctional complex. We found marked alteration in the activation profile of the small GTPase Rac1 upon deletion of the cytoplasmic JMD. Rac1 is a regulator of actin nucleation and polymerization at the leading edge of migrating cells, and evidence has been accumulating that implicates Rac1 in the stabilization of cadherin intercellular junctions (Braga et al., 1997; Nobes and Hall, 1999). We found that in wild-type cadherin-11 L-cells, the level of Rac1 activity was low. In contrast, we found that the level of Rac1 activity was markedly higher in the cadherin-11 ΔJMD L-cells, similar to levels in L-cells lacking cadherins. These results suggest that in wild-type cadherin-11 L-cells, Rac1 activity is actively down-modulated, whereas cadherin-11 ΔJMD L-cells fail to down-modulate Rac1 activity. Cadherin regulation of small Rho GTPases has mainly been examined in the context of E-cadherin and epithelial cells. Notably, E-cadherin engagement has been shown to increase Rac1 activity (Noren et al., 2001; Kovacs et al., 2002). This is consistent with the finding that Tiam 1, an activator for Rac1, localizes to sites of cell-to-cell adhesion in epithelial cells (Sander et al., 1998). Our studies now demonstrate down-modulation of Rac1 activity in wild-type cadherin-11 L-cells and suggest the opposite effect on Rac1 activity for cadherin-11 in fibroblasts compared with E-cadherin in epithelial cells. Our observation parallels a recent report that shows N-cadherin dependent down-regulation of Rac1 activity upon cell-to-cell contact in myoblasts (Charrasse et al., 2002). Given the differential regulation of Rac1 activity in wild-type and ΔJMD cadherin-11 L-cells, we now suggest a critical role of the cadherin-11cytoplasmic JMD in down-modulating Rac1 activity.
The consequence of diminished Rac1 activity upon wild-type cadherin-11 engagement seems likely, at least in part, to account for the dynamic remodeling of cell-to-cell contacts. In vitro, keratinocytes microinjected with dominant negative Rac1 or epithelial cells treated with cytotoxic necrotizing factor 1, a toxin that causes sustained depletion of Rac1, are unable to maintain tight adhesion and cell-to-cell contacts become loose (Braga et al., 1997; Doye et al., 2002). Correspondingly, overexpression of a constitutively active form of Rac1 results in tight adhesion and accumulation of actin at sites of intercellular junctions (Takaishi et al., 1997). In our system, in which Rac1 activity was high in the cadherin-11 ΔJMD L-cells, Rac1 activity may localize to the junctional complex and exert its effect on stabilizing cadherin-catenin complexes and actin reorganization in a manner dependent on cell-to-cell contacts. Thus, in these cells high Rac1 activity may reduce turnover of α-catenin at AJs and cadherin-11-dependent intercellular motility. The downstream effects of Rac1 (Kuroda et al., 1998) may explain our results from FRAP studies in which α-catenin exchange at AJs was dramatically reduced in the cadherin-11 ΔJMD L-cells compared with wild-type cadherin-11 L-cells. Based on these results, we propose that the cadherin-11 cytoplasmic JMD is critical for down-regulating Rac activity and allows cadherin-11 driven motility to become effective.
Besides interfering with RhoGTPases, the cadherin-11 JMD may control the stability of the cadherin-11-β-catenin-α-catenin complex by the recruitment of kinases and/or protein tyrosine phosphatases (PTPases) to AJs. The integrity and function of the cadherin-β-catenin-α-catenin complex is in part regulated by phosphorylation of its components (Nelson and Nusse, 2004). Therefore, the difference in α-catenin turnover between the wild-type and ΔJMD cadherin-11 L-cells, as revealed in the FRAP experiments, might be due to differential recruitment of kinases and/or PTPases to the junctional complex. Indeed, recent studies show that p120ctn that is bound to the N-cadherin cytoplasmic JMD can associate with Fer, a cytoplasmic kinase that has been implicated in the phosphorylation of β-catenin as well as PTP1B (Xu et al., 2002, 2004).
Cytoplasmic JMD controlled cellular rearrangements may also involve cadherin-11 internalization, recycling, or its degradation. The cadherin cytoplasmic JMD and its binding partner p120ctn have been found to regulate cadherin levels at the cell surface by controlling the entry of cadherins into endocytic pathways (Ireton et al., 2002; Davis et al., 2003; Xiao et al., 2003). In the absence of p120ctn, cadherins are rapidly internalized and degraded. Thus, molecular interactions at the cadherin-11 cytoplasmic JMD may regulate vesicular transport and/or the cytoplasmic fate of cadherin-11, thereby controlling AJ turnover and facilitating remodeling of cell-to-cell contacts.
In conclusion, our data show that cadherin-11 is an intercellular motility factor that actively promotes cellular rearrangements. Furthermore, we define a new function for the cadherin-11 cytoplasmic JMD in regulating α-catenin turnover at AJs and cadherin-11 driven intercellular motility.
Supplementary Material
Acknowledgments
We are grateful to Hui-Ya Gilbert and Jean Lai for expert technical assistance with confocal microscopy. We thank Drs. P. Peters and V. Hsu for useful discussions. We thank Dr. Reichhardt (University of California, San Francisco) for providing us with the pEGFP-αN-catenin construct. We received grant support from National Institutes of Health Grant AR48114 (to M.B.B.), the Arthritis Foundation (to H.P.K.), National Institutes of Health Grant CA-86712 (to M. Hemler and C.S.S.), the Claudia Adams Barr Program in Cancer Research (to C.S.S.), and the Medical Foundation Charles A. King Trust (to C.S.S.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0745) on March 8, 2006.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, C. L., Chen, Y. T., Smith, S. J., and Nelson, W. J. (1998). Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell. Biol. 142, 1105-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono, S., Nakagawa, S., Reynolds, A. B., and Takeichi, M. (1999). p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J. Cell. Biol. 145, 551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki, L., et al. (2001). Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proc. Natl. Acad. Sci. USA 98, 2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers, A., David, R., and Wedlich, D. (2001). Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128, 3049-3060. [DOI] [PubMed] [Google Scholar]
- Braga, V. M., Machesky, L. M., Hall, A., and Hotchin, N. A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell. Biol. 137, 1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher, W. M., and Gumbiner, B. M. (1994). Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J. Cell. Biol. 126, 519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse, S., Meriane, M., Comunale, F., Blangy, A., and Gauthier-Rouviere, C. (2002). N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell. Biol. 158, 953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. A., Ireton, R. C., and Reynolds, A. B. (2003). A core function for p120-catenin in cadherin turnover. J. Cell. Biol. 163, 525-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doye, A., Mettouchi, A., Bossis, G., Clement, R., Buisson-Touati, C., Flatau, G., Gagnoux, L., Piechaczyk, M., Boquet, P., and Lemichez, E. (2002). CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111, 553-564. [DOI] [PubMed] [Google Scholar]
- Drees, F., Pokutta, S., Yamada, S., Nelson, W. J., and Weis, W. I. (2005). Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123, 903-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H. E., Behrens, J., Sommer, T., and Birchmeier, W. (2002). Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell. Biol. 4, 222-231. [DOI] [PubMed] [Google Scholar]
- Getsios, S., Chen, G. T., Stephenson, M. D., Leclerc, P., Blaschuk, O. W., and MacCalman, C. D. (1998). Regulated expression of cadherin-6 and cadherin-11 in the glandular epithelial and stromal cells of the human endometrium. Dev. Dyn. 211, 238-247. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B. M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345-357. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell. Biol. 6, 622-634. [DOI] [PubMed] [Google Scholar]
- Herrenknecht, K., Ozawa, M., Eckerskorn, C., Lottspeich, F., Lenter, M., and Kemler, R. (1991). The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc. Natl. Acad. Sci. USA 88, 9156-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J.M.G., Mandlebrot, D. A., Shaw, S. K., Russell, G. J., Murphy, E. A., Chen, Y. T., Nelson, W. J., Parker, C. M., and Brenner, M. B. (1998). Direct and regulated interaction of integrin αEβ7 with E-cadherin. J. Cell. Biol. 140, 197-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton, R. C., et al. (2002). A novel role for p120 catenin in E-cadherin function. J. Cell. Biol. 159, 465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou, T. S., Stewart, D. B., Stappert, J., Nelson, W. J., and Marrs, J. A. (1995). Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc. Natl. Acad. Sci. USA 92, 5067-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y., Matsunami, H., Inoue, T., Shimamura, K., Uchida, N., Ueno, T., Miyazaki, T., and Takeichi, M. (1995). Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev. Biol. 169, 347-358. [DOI] [PubMed] [Google Scholar]
- Kobielak, A., Pasolli, H. A., and Fuchs, E. (2004). Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell. Biol. 6, 21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, E. M., Ali, R. G., McCormack, A. J., and Yap, A. S. (2002). E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708-6718. [DOI] [PubMed] [Google Scholar]
- Kuroda, S., et al. (1998). Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science 281, 832-835. [DOI] [PubMed] [Google Scholar]
- Lee, C. H., and Gumbiner, B. M. (1995). Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev. Biol. 171, 363-373. [DOI] [PubMed] [Google Scholar]
- Locascio, A., and Nieto, M. A. (2001). Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev. 11, 464-469. [DOI] [PubMed] [Google Scholar]
- Markus, M. A., Reichmuth, C., Atkinson, M. J., Reich, U., Hoffmann, I., Balling, R., Anderer, U., and Hofler, H. (1999). Cadherin-11 is highly expressed in rhabdomyosarcomas and during differentiation of myoblasts in vitro. J. Pathol. 187, 164-172. [DOI] [PubMed] [Google Scholar]
- Nelson, W. J., and Nusse, R. (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska, P., Godt, D., and Tepass, U. (1999). DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J. Cell. Biol. 144, 533-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C. D., and Hall, A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell. Biol. 144, 1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N. K., Niessen, C. M., Gumbiner, B. M., and Burridge, K. (2001). Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305-33308. [DOI] [PubMed] [Google Scholar]
- Okazaki, M., Takeshita, S., Kawai, S., Kikuno, R., Tsujimura, A., Kudo, A., and Amann, E. (1994). Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 269, 12092-12098. [PubMed] [Google Scholar]
- Ozawa, M. (2003). p120-independent modulation of E-cadherin adhesion activity by the membrane-proximal region of the cytoplasmic domain. J. Biol. Chem. 278, 46014-46020. [DOI] [PubMed] [Google Scholar]
- Ozawa, M., Baribault, H., and Kemler, R. (1989). The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 8, 1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, M., and Kemler, R. (1992). Molecular organization of the uvomorulin-catenin complex. J. Cell. Biol. 116, 989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, M., and Kemler, R. (1998). The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J. Cell. Biol. 142, 1605-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, F., and D'Souza-Schorey, C. (2003). Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J. Biol. Chem. 278, 17395-17400. [DOI] [PubMed] [Google Scholar]
- Pishvaian, M. J., Feltes, C. M., Thompson, P., Bussemakers, M. J., Schalken, J. A., and Byers, S. W. (1999). Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 59, 947-952. [PubMed] [Google Scholar]
- Sander, E. E., van Delft, S., ten Klooster, J. P., Reid, T., van der Kammen, R. A., Michiels, F., and Collard, J. G. (1998). Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell. Biol. 143, 1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi, K., Sasaki, T., Kotani, H., Nishioka, H., and Takai, Y. (1997). Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell. Biol. 139, 1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson, M. A., Anastasiadis, P. Z., Daniel, J. M., Ireton, R. C., Wheelock, M. J., Johnson, K. R., Hummingbird, D. K., and Reynolds, A. B. (2000). Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell. Biol. 148, 189-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, K., van Bokhoven, A., van Leenders, G. J., Ruijter, E. T., Jansen, C. F., Bussemakers, M. J., and Schalken, J. A. (2000). Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650-3654. [PubMed] [Google Scholar]
- Uemura, T., Oda, H., Kraut, R., Hayashi, S., Kotaoka, Y., and Takeichi, M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659-671. [DOI] [PubMed] [Google Scholar]
- Valencia, X., et al. (2004). Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J. Exp. Med. 200, 1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin, V., Bauer, C., Yin, M., and Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209-219. [DOI] [PubMed] [Google Scholar]
- Xiao, K., Allison, D. F., Buckley, K. M., Kottke, M. D., Vincent, P. A., Faundez, V., and Kowalczyk, A. P. (2003). Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G., Arregui, C., Lilien, J., and Balsamo, J. (2002). PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J. Biol. Chem. 277, 49989-49997. [DOI] [PubMed] [Google Scholar]
- Xu, G., Craig, A. W., Greer, P., Miller, M., Anastasiadis, P. Z., Lilien, J., and Balsamo, J. (2004). Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J. Cell. Sci. 117, 3207-3219. [DOI] [PubMed] [Google Scholar]
- Yamada, S., Pokutta, S., Drees, F., Weis, W. I., and Nelson, W. J. (2005). Deconstructing the cadherin-catenin-actin complex. Cell 123, 889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A. S., Niessen, C. M., and Gumbiner, B. M. (1998). The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell. Biol. 141, 779-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.