Abstract
The neural cell adhesion molecule (NCAM) plays a pivotal role in the development of the nervous system, promoting neuronal differentiation via homophilic (NCAM–NCAM) as well as heterophilic (NCAM-fibroblast growth factor receptor [FGFR]) interactions. NCAM-induced intracellular signaling has been shown to affect and be dependent on the cytoplasmic Ca2+ concentration ([Ca2+]i). However, the molecular basis of this remains unclear. In this study, we determined [Ca2+]i regulating mechanisms involved in intracellular signaling induced by NCAM. To mimic the effect of homophilic NCAM interaction on [Ca2+]i in vitro, we used a peptide derived from a homophilic binding site of NCAM, termed P2, which triggers signaling cascades similar to those activated by NCAM–NCAM interaction. We found that P2 increased [Ca2+]i in primary hippocampal neurons. This effect depended on two signaling pathways. The first pathway was associated with activation of FGFR, phospholipase Cγ, and production of diacylglycerol, and the second pathway involved Src-family kinases. Moreover, NCAM-mediated Ca2+ entry required activation of nonselective cation and T-type voltage-gated Ca2+ channels. These channels, together with the Src-family kinases, were also involved in neuritogenesis induced by physiological, homophilic NCAM interactions. Thus, unanticipated mechanisms of Ca2+ homeostasis are shown to be activated by NCAM and to contribute to neuronal differentiation.
INTRODUCTION
The neural cell adhesion molecule (NCAM) belongs to the Ig superfamily and has a variety of functions in the development and maintenance of the nervous system (reviewed in Povlsen et al., 2003; Hinsby et al., 2004). The extracellular domain of NCAM consists of five Ig modules (IgI–V) and two fibronectin type III homology modules (F3, I and II). Importantly, NCAM promotes neurite outgrowth via homophilic (NCAM–NCAM) as well as heterophilic (NCAM–fibroblast growth factor receptor [FGFR]) interactions (Kiselyov et al., 2005), which activate a number of intracellular signaling cascades (reviewed in Walmod et al., 2004). Recently, a novel mechanism of homophilic NCAM binding has been proposed, in which the IgI and IgII modules mediate dimerization of NCAM molecules situated on the same cell surface (cis interactions), whereas trans interactions between the IgI and IgIII, IgII and IgII, and the IgII and IgIII modules of NCAM determine cell–cell adhesion (Soroka et al., 2003).
Although NCAM-induced intracellular signaling has been demonstrated to affect and be dependent on cytoplasmic Ca2+ concentration ([Ca2+]i) (reviewed in Kiryushko et al., 2004), data concerning the role of Ca2+ in NCAM-mediated signal transduction are limited. Neurite outgrowth induced by homophilic binding of cell adhesion molecules (CAMs) in dorsal root ganglia neurons has been shown to depend on a Ca2+ influx via voltage-dependent Ca2+ channels (VDCCs) (Doherty et al., 1991; Archer et al., 1999). It has also been demonstrated by spectrofluorimetry that NCAM antibodies elicit a long-lasting increase of [Ca2+]i in PC12 cells (Schuch et al., 1989) and primary cerebellar neurons (Von Bohlen Und Halback et al., 1992). However, the molecular mechanisms of these events have not been elucidated.
The aim of this study was to investigate [Ca2+]i -regulating mechanisms involved in the intracellular signaling triggered by NCAM, and their importance for NCAM-induced neurite outgrowth. Previously, either cocultures of neurons grown on a monolayer of NCAM-expressing cells or stimulation with soluble NCAM have been used to mimic homophilic NCAM binding in vitro (Kiryushko et al., 2004). However, the coculture approach has significant limitations because of difficulty in presenting NCAM to appropriate ligands in a quantitative manner. Also, stimulation with soluble NCAM is hampered due to NCAM homodimerization via homophilic interaction. These factors impede studies of NCAM effects on rapidly changing cellular parameters (e.g., [Ca2+]i).
To mimic the effect of homophilic NCAM binding on [Ca2+]i in vitro, we therefore used a synthetic peptide ligand of NCAM, termed P2. The peptide represents a part of the IgII module involved in homophilic NCAM interactions, and it binds to the IgI module of NCAM with a Kd similar to that of NCAM IgII (Soroka et al., 2002). The tetrameric form of this peptide (P2d) in vitro induces neurite outgrowth in the absence of homophilic trans NCAM binding (Soroka et al., 2002) and affects NCAM-mediated cell adhesion (Li et al., 2005). Moreover, the peptide triggers intracellular signaling cascades similar to those activated by NCAM itself (Soroka et al., 2002). Therefore, P2d was used as a tool to study which [Ca2+]i-regulating mechanisms are involved in intracellular signaling induced by NCAM.
MATERIALS AND METHODS
Materials
Tetrodotoxin, the inhibitor of voltage-gated sodium channels, and Hank's balanced salt solution (HBSS) were from Sigma-Aldrich (Copehnagen, Denmark). The receptor tyrosine kinase inhibitors lavendustin A and B; blockers of voltage-gated calcium channels of T-type (flunarizine), L-type (nifedipine), N-type (ω-conotoxin MVIIA), and P/Q-type (ω-agatoxin TK); and a blocker of Ca2+ uptake into the endoplasmic reticulum (ER) stores, thapsigargin, were all from Calbiochem (Merck, Darmstadt, Germany). The phospholipase C (PLC) inhibitor U-73122 and the diacylglycerol (DAG)-lipase inhibitor RHC-80267 were from BIOMOL Research Laboratories (Plymouth Meeting, PA). A T-type Ca2+ channels inhibitor mibefradil (dihydrochloride salt) was a gift from F. Hoffmann-La Roche (Basel, Switzerland). P2d (GRILARGEINFK) and scrambled P2d (NLFEKGRRAIGI) peptides were produced as described by Soroka et al. (2002).
Primary Cultures of Rat Hippocampal Neurons and Analysis of Neurite Outgrowth
Hippocampal neurons were prepared from embryonic day (E) 19 Wistar rat embryos as described by Maar et al. (1997). Neurons were seeded at a density of 10,000 cells/cm2 in eight-well Lab-Tek tissue culture chambers with a growth surface of Permanox plastic (NUNC, Roskilde, Denmark). After plating, cultures were maintained in Neurobasal medium as described previously (Soroka et al., 2002). For long-term cultures, cytosine arabinofuranoside (final concentration 5 μM; Sigma-Aldrich) was added after 2 d in vitro to inhibit glial cell proliferation. Analysis of neurite outgrowth was performed as described previously (Soroka et al., 2002).
Coculture of Hippocampal Neurons and L929 Fibroblasts without or with NCAM Expression
Hippocampal neurons were plated on top of a confluent monolayer of L929 fibroblasts without or with NCAM expression (Kolkova et al., 2000). Inhibitors were added to the medium immediately after plating. Cocultures were maintained during 24 h at 37°C, 5% CO2 in Neurobasal-A medium supplemented with 2% (vol/vol) B27, 5% (vol/vol) fetal calf serum, and 2 mM GlutaMAX (all reagents from Invitrogen, Carlsbad, CA).
Fluorometric Ca2+ Measurements
Dye loading and experiments were performed in modified HBSS at room temperature (22–24°C). A modified HBSS used in experiments with physiological extracellular Ca2+ was produced by adjusting Ca2+ concentration to 2 mM, pH 7.4. A low calcium HBSS was obtained by adjusting the Ca2+ concentration to 200 μM and adding CdCl2 to the solution to a final concentration of 200 μM. Primary neurons were seeded on eight-well poly-l-lysine–coated Lab-Tek chambered coverglass slides (NUNC) at a density of 50,000 cells/well and grown for 7–10 d. Attached cells were loaded with a Ca2+-sensitive dye fura-2 acetoxymethyl ester (AM) (5 μM; Invitrogen) or fluo-4 AM (2 μM; Invitrogen). To eliminate the effect of synaptically induced Ca2+ fluxes, all experiments were performed in the presence of 1 μM tetrodotoxin. The regions of interest were positioned on somata of the neurons, which were identified by their morphological characteristics. Data were collected from at least three sets of cultures obtained from different animals; each experiment provided simultaneous measurements from up to eight neurons.
Microscopy and Data Analysis
Immunostaining of primary neurons and image scanning were performed as described by Soroka et al. (2002). Rabbit anti-rat-growth–associated protein (GAP)-43 polyclonal antibodies (Cell Signaling Technology, Beverly, MA) were used.
Calcium imaging employing fura-2 AM was performed using a Sensicam 12-bit cooled charge-coupled device camera (PCO, Keilheim, Germany) and a J&M monochromator (J&M, Aalen, Germany) connected to a Zeiss Axiovert 135 TV microscope (Zeiss Fluar oil immersion UV objective, 40×, 1.3 numerical aperture). The cellular fluorescence signals were corrected for the background fluorescence, which was measured in a separate region in the vicinity of the cell. Ratio images (340/380 nm excitation; 510 LP emission) were collected after background subtraction at 2- to 5-s intervals. The calibration constants and the estimated [Ca2+]i were calculated from a fluorescence ratio, R, as described by Rønn et al. (2002). Data acquisition and analysis were performed using the Imaging Workbench software (Molecular Devices, Sunnyvale, CA).
Immunoblotting Assay
Hippocampal neurons were seeded in 60-mm tissue culture dishes (106 cells) and grown for 4 h before treatment. Immunoblotting was performed as described previously (Kolkova et al., 2000). Rabbit anti-phospho-PLCγ1 antibodies (diluted 1:300; Cell Signaling Technology) or rabbit anti-phospho-Src antibodies (diluted 1:600; Cell Signaling Technology) were used. Protein bands were visualized using the enhanced chemiluminescence substrate Western Dura (Pierce Chemical, Rockford, IL) and processed with the GenTools software package (Syngene, Cambridge, United Kingdom). To estimate the total amount of PLCγ1 or actin, membranes were stripped and reprobed with an anti-PLCγ1 or anti-actin antibodies (both developed in rabbit; Cell Signaling Technology and Sigma-Aldrich, respectively).
Statistics and Graphical Presentation
Statistics and graphical presentations were carried out using the Origin version 6.0 software package (OriginLab, Northampton, MA). Statistical evaluations were performed using a two-sided Student's t test. The results are given as mean ± SEM. Unless otherwise stated asterisks indicate the statistical significance of *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control.
RESULTS
P2d Induces Both Rapid Neurite Extension and a [Ca2+]i Elevation in Primary Hippocampal Neurons
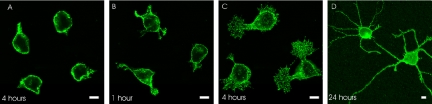
It has previously been shown by Soroka et al., (2002) that tetrameric P2 (P2d), but not a scrambled variant of P2d (scrP2d) induces differentiation of primary hippocampal neurons. We determined the time course of the P2d-triggered neurite extension. Neurons stimulated with scrP2d did not change morphology even after 4 h of treatment (Figure 1A). In contrast, P2d induced the formation of initial protrusions already 1 h after application (Figure 1B), and after 4 h, the formation of primary neurites was observed (Figure 1C). After 24 h, the P2d-treated neurons had an average neurite length of ∼100 μm per cell (Figure 1D).
Figure 1.
P2d induces a fast neuritogenic response from primary hippocampal neurons. (A–D) Confocal images of hippocampal neurons treated for the indicated lengths of time with 5 μM scrambled P2d (A) or 5 μM P2d (B–D). The cells were immunostained for GAP-43. Representative neurons are shown. Bars, 5 μm.
Homophilic binding of CAMs is known to increase the cytoplasmic Ca2+ level (Archer et al., 1999). We therefore tested whether P2d affected [Ca2+]i in cultured hippocampal neurons at various times after plating. Indeed, P2d induced Ca2+ responses, although of differing amplitudes, in hippocampal neurons grown for 6 h and 7–10 d in vitro, whereas no change of [Ca2+]i was observed after application of scrambled P2d (Figure 2A). Thus, only the neuritogenically active peptide was capable of elevating the cytoplasmic Ca2+ level, and the magnitude of the [Ca2+]i rise depended on the time neurons had been cultured. An amplitude of P2d-induced Ca2+ responses also depended on the age of the animals, from which primary neurons were obtained, being at 6 h in vitro approximately twofold higher in the cells isolated from P5 than in the cells isolated from E19 animals (Figure 2A). In subsequent experiments, we used P2d at a concentration of 35 μM (calculated per monomer), because this concentration both induced neurite outgrowth (Soroka et al., 2002) and a prominent Ca2+ response (Figure 2B). However, lower concentrations of P2d also elevated [Ca2+]i with a threshold of activation observed at ∼10 μM P2d (Figure 2B). In the presence of the peptide, the P2d-induced Ca2+ responses sustained for at least 20 min (Figure 2B, inset). When P2d was washed out, a slow recovery of [Ca2+]i to the resting level was observed (Figure 2B, inset). Hippocampal cultures grown for 7 d in vitro were used for Ca2+ fluorometry because of their robust high-magnitude Ca2+ responses to P2d. Selected experiments were also reproduced in cultures grown for 6 h in vitro, yielding similar results (our unpublished data).
Figure 2.
P2d increases [Ca2+]i in primary hippocampal neurons. (A) Ca2+ responses induced by P2d and scrP2d (30 μM both) in primary hippocampal neurons. (B) Dose-response relationship for P2d-triggered [Ca2+]i rise in primary hippocampal neurons cultured for 7–10 d. Inset, Ca2+ responses induced by P2d present either for 15 min (open squares) or for 3 min (filled squares). DIV, days in vitro. In all fluorometric experiments, number of cells n = 40–60.
The P2d-induced Rise of [Ca2+]i Involves VDCCs as Well as [Ca2+]i Stores
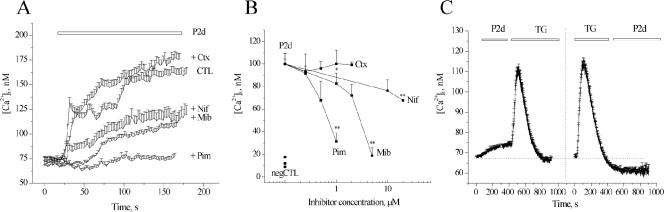
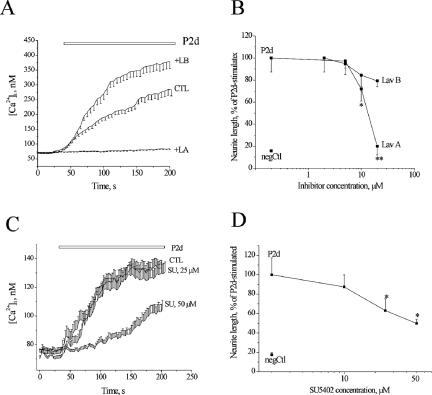
Activity of VDCCs has been shown to be important for NCAM-induced neurite outgrowth (Doherty et al., 1991). Using specific inhibitors, we checked whether this type of channels was involved in the P2d-induced Ca2+ response. Blocking L-type VDCCs with nifedipine or T-type VDCC with mibefradil led to a partial (∼50%) decrease in the amplitude of the P2d-evoked [Ca2+]i elevation (Figure 3A). The partial inhibition of the Ca2+ response by mibefradil might be due to this drug also being a potent blocker of calcium-activated chloride channels (Nilius and Droogmans, 2003), which produce hyperpolarization of hippocampal neurons at this developmental stage (Stein et al., 2004). However, a more selective T-type VDCCs inhibitor, pimozide, completely blocked the Ca2+ response to P2d (Figure 4A). In contrast, inhibition of N-type VDCC by ω-conotoxin did not influence the magnitude of the P2d-induced [Ca2+]i elevation (Figure 3A). Neither did the P/Q-type Ca2+ channels inhibitor agatoxin have any effect (our unpublished data). In agreement with the fluorometric data, P2d-triggered neurite outgrowth was not affected by agatoxin (our unpublished data) or ω-conotoxin (Figure 3B). Nifedipine caused a dose-dependent inhibition of the P2d-induced neuritogenic response with a 40% reduction in average neurite length at a concentration of 20 μM (Figure 3B), whereas mibefradil and pimozide almost completely blocked P2d-induced neurite extension at concentrations close to their 2× K1/2 values (2.5 and 2.2 μM, respectively). This suggested that the activity of T-type, but not L-type, Ca2+ channels was crucial for neurite induction by P2d.
Figure 3.
P2d-induces Ca2+ entry via VDCCs and triggers Ca2+ release from intracellular stores. (A and B) Effects of different blockers of VDCCs on P2d-induced [Ca2+]i rise and neurite outgrowth, respectively. CTL, no inhibitor added; Nif, nifedipine (10 μM), a blocker of L-type VDCCs; Mib, mibefradil and Pim, pimozide (5 μM both), blockers of T-type VDCCs; and Ctx, conotoxin (2 μM), a blocker of N-type VDCCs. Here and henceforth, 100% indicates neurite outgrowth from cultures stimulated with P2d in the absence of inhibitors, and negCTL indicates neurite outgrowth from nonstimulated cultures. (C) PC12 cells were challenged with P2d either before (left; n = 22) or after (right; n = 18) application of an irreversible inhibitor of Ca2+ uptake into the ER stores, thapsigargin (TG, 200 nM), in a low Ca2+ medium. In all fluorometric experiments, n = 20–40.
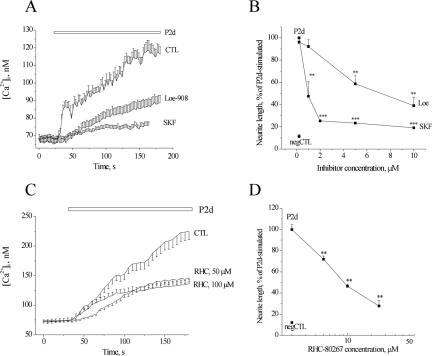
Figure 4.
Nonselective cation channels and DAG-lipase participate in P2d-triggered signaling. (A and B) Effect of two different inhibitors of nonselective cation entry, SKF-96565 and Loe908, on P2d-evoked [Ca2+]i rise (A) and neurite elongation (B). (C and D) Dose-dependent inhibition of P2d-triggered Ca2+ response (C) and neurite outgrowth (D) by a blocker of DAG-lipase, RHC-80267. In all fluorometric experiments, n = 20–40.
Because Ca2+ retained within the ER also might contribute to the P2d-triggered [Ca2+]i rise, we checked, whether application of the peptide led to a Ca2+ release from the intracellular ER stores. Cultured hippocampal neurons have been reported to possess overlapping Ca2+-(ryanodine) and inositol trisphosphate-sensitive stores; however, no detectable Ca2+ response could be induced in the neurons by application of drugs activating the respective receptors on the surface of the stores (Irving and Collingridge, 1998). Therefore, we used the NCAM-expressing PC12E2 cell line known to possess a high amount of releasable Ca2+ in the stores. To estimate the P2d-induced Ca2+ release from the ER, the stores were challenged with P2d in a low Ca2+ medium (see Materials and Methods for composition). A long-lasting low-amplitude [Ca2+]i transient was observed (Figure 3C, left). After the P2d-induced response, thapsigargin (TG), a high-affinity blocker of Ca2+ pumps of the ER, still was able to release Ca2+ from the lumen of ER, indicating that P2d did not deplete the stores (Figure 3C, left). When TG was applied before P2d, thereby emptying the stores, the cells were no longer able to mount a Ca2+ response to P2d (Figure 3C, right), indicating that P2d indeed induced a Ca2+ release from the ER.
The P2d-induced [Ca2+]i Rise Involves Nonselective Cation Channels and Requires Activation of the FGFR–PLCγ–DAG Signaling Pathway
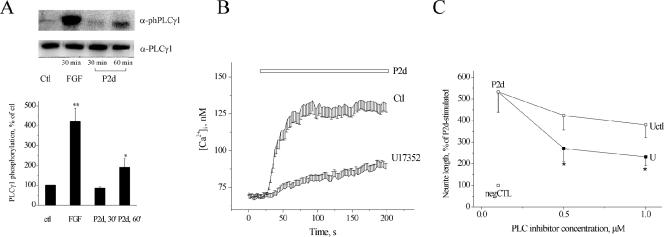
We next tested, whether any other types of ion channels were involved in the P2d-induced Ca2+ response. NCAM homophilic binding results in the phosphorylation of FGFR, leading to an activation of the phosphoinositide-specific PLCγ and to a formation of DAG, which can further be hydrolyzed by DAG-lipase to 2-arachidonoylglycerol (2-AG), and subsequently to arachidonic acid (AA) (reviewed in Kiryushko et al., 2004). All these messengers activate nonselective cation channels (NSCCs) (reviewed in Hardie 2003). We found that two different inhibitors of NSCCs, Loe-908 and SKF-96562, decreased the P2d-induced Ca2+ response (Figure 4A) as well as neurite elongation (Figure 4B) in a dose-dependent manner. This indicated that the nonselective Ca2+ entry, possibly alongside with Ca2+ entry via T-type VDCCs, was responsible for initiation of the Ca2+ response to P2d. To test, whether DAG and/or its downstream products were involved in P2d-induced NSCC activation, DAG-lipase was blocked by the specific inhibitor RHC-80267, resulting in a dose-dependent decrease of P2d-triggered Ca2+ transients (Figure 4C) and neurite outgrowth (Figure 4D). An NCAM-induced signaling cascade leading to DAG generation was most probably associated with PLCγ (Hinsby et al., 2004, and references therein). Indeed, in hippocampal neurons, P2d phosphorylated PLCγ1 on the Tyr783 residue (Figure 5A), an event known to be both necessary and sufficient for PLCγ1 activation (Kim et al., 1991). PLCγ2 was not expressed in detectable amounts in hippocampal neurons (Kiryushko et al., 2006). Thus, P2d launched intracellular signaling via PLCγ1, and the hydrolysis of DAG to 2-AG and/or AA contributed to the induction of the cellular response to the peptide.
Figure 5.
P2d-induced cellular responses depend on PLCγ. (A) Top, increase in Tyr783 phosphorylation of PLCγ1 induced by FGF2 (FGF; 10 nM) and P2d (P2d; 5 μM), representative immunoblot of four individual experiments is shown. Bottom, results from four independent experiments. (B and C) Effects of the inhibitor of phosphoinositide-specific PLCs (U17352) on P2d-induced Ca2+ responses (B, inhibitor concentration 1 μM) and neurite extension (C). U, U17352; Uctl, U17350 (a control compound for U17352).
Interestingly, blocking of DAG-lipase did not inhibit the P2d-induced Ca2+ response completely (Figure 4, C and D), suggesting either that DAG itself also plays a role or that other signaling cascade(s) also may contribute to the P2d-induced Ca2+ response. In that case, inhibition of PLCγ should not result in a complete blocking of the P2d-induced Ca2+ rise. Indeed, U17352, an inhibitor of phosphoinositide-specific PLCs, did not abolish P2d-induced Ca2+ elevation or neurite outgrowth, although decreasing both by ∼70% (Figure 5, B and C). This indicated that P2d might activate other relevant signaling cascade(s) besides that associated with PLC.
NCAM-induced intracellular signaling has previously been shown to be mediated by FGFR, a member of the receptor tyrosine kinase (RTK) family (Saffell et al., 1997; Kiselyov et al., 2005). An antagonist of RTKs and protein tyrosine kinases, lavendustin A, but not its inactive analog lavendustin B, abolished P2d-induced Ca2+ entry and neurite outgrowth (Figure 6, A and B, respectively). The observed effect of lavendustin A could be partially attributed to the inhibition of FGFR1, a ubiquitous FGFR type in primary neurons, because a specific blocker of FGFR1 (SU5402) decreased both [Ca2+]i rise (Figure 6C) and neurite extension induced by P2d (Figure 6D). However, even at the maximal antagonist concentration the inhibition was only ∼50%, suggesting that another signaling mechanism might be involved in the induction of cellular responses by P2d.
Figure 6.
P2d-triggered signaling is mediated by RTKs and/or PTKs but only partially depends on FGFR. Effect of the inhibitors of RTK (lavendustin A) and FGFR1 (SU5402) on P2d-induced [Ca2+]i rise (A and C, respectively) and neurite outgrowth (B and D, respectively). LA, lavendustin A; LB, lavendustin B (a control compound to LA); SU, SU5402; n = 20–40.
Src-Family Kinases Are Involved in the P2d-induced Intracellular Signaling
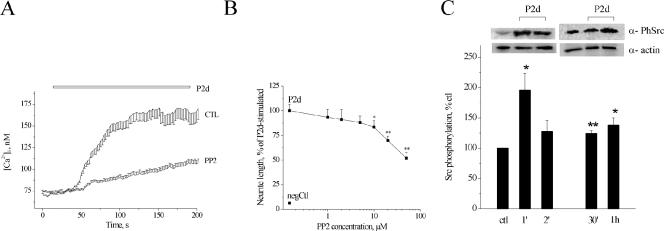
Because lavendustin A inhibits both RTKs and the Src-family kinases known to be involved in NCAM-induced signaling (reviewed in Walmod et al., 2004), we tested, whether Src-family kinases participated in the P2d-induced Ca2+ signaling using a specific inhibitor, PP2. Indeed, PP2 caused a significant reduction in both [Ca2+]i rise (Figure 7A) and neurite outgrowth (Figure 7B) in response to P2d. We therefore checked whether P2d increased the phosphorylation of Src-family kinases on the Tyr416 residue shown to be involved in the activation of these enzymes. As seen in Figure 7C, the peptide induced phosphorylation of the Src-family kinases with a maximum activation observed already 1 min after application. The increase in the amount of the phosphorylated enzyme form was also observed 30 min and 1 h after peptide application. Thus, P2d as well as NCAM itself (Beggs et al., 1997) triggered Src-mediated signaling.
Figure 7.
Src-family kinases are involved in P2d-induced cellular responses. (A and B) Effect of the inhibitor of Src-family kinases (PP2) on P2d-induced Ca2+ responses (A) and neurite outgrowth (B). (C) Top, increase in Tyr416 phosphorylation of Src-family kinases induced by P2d (5 μM), representative immunoblots of three or four individual experiments are shown. Bottom, results from three or four independent experiments.
Src-Family Kinases, T-type VDCC, and NSCC Are Involved in the NCAM-induced Neurite Outgrowth
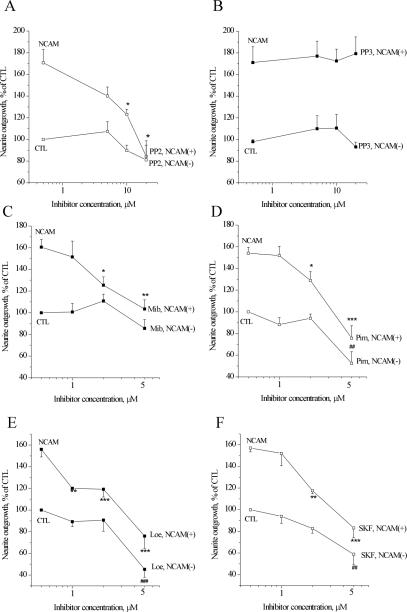
We next checked, whether the mechanisms of Ca2+ homeostasis involved in the signaling triggered by P2d were of importance for physiological NCAM-induced differentiation. In this assay, primary hippocampal neurons are grown on monolayers of fibroblasts expressing NCAM, a condition known to mimic physiological NCAM homophilic trans interactions. Under these circumstances, NCAM is known to induce neurite extension from a variety of neuronal cell types (reviewed in Hinsby et al., 2004). As seen in Figure 8, hippocampal neurons grown on monolayers of NCAM-expressing fibroblasts extended ∼60% longer neurites (NCAM control) than neurons grown in coculture with fibroblasts without NCAM expression (negative control). NCAM-specific neurite outgrowth could be abolished by the blocker of Src-family kinases PP2 (Figure 8A), whereas the inactive PP2 analog PP3 had no effect (Figure 8B).
Figure 8.
Src-family kinases, VDCCs, and NSCCs are involved in the NCAM-induced neurite outgrowth. (A and B) Effect of the inhibitors of Src-family kinases PP2 (A) and its inactive analog PP3 (B) on NCAM-induced neurite outgrowth. (C and D) Effect of the inhibitors of T-type VDCCs mibefradil (Mib) (C) and pimozide (Pim) (D) on NCAM-induced neurite outgrowth. (E and F) Effect of the inhibitors of NSCCs Loe908 (Loe) (E) and SKF-96365 (SKF) (F) on NCAM-induced neurite outgrowth. NCAM and control (CTL_, neurite outgrowth from neurons grown on monolayers of fibroblasts with (NCAM+) and without (NCAM–) NCAM expression, respectively, in the absence of inhibitors. Asterisk (*) indicates significance compared with NCAM; pound (#) indicates significances compared with CTL.
Blockers of both T-type VDCCs (mibefradil, Figure 8C; and pimozide, Figure 8D) and NSCC (Loe908, Figure 8E; and SKF-96365, Figure 8F) all significantly reduced NCAM-specific neurite extension when applied in low (∼K1/2) concentrations. At concentrations close to their 2 × K1/2, the inhibitors also affected neurite extension from the neurons grown on fibroblasts without NCAM expression. Thus, the activity of T-type VDCCs and NSCCs was important for NCAM-dependent neuritogenesis, but it probably also played a role in the neurite outgrowth response induced by other cell surface-associated neuritogenic cues.
DISCUSSION
In this study, we have investigated [Ca2+]i regulating mechanisms involved in NCAM signaling using a synthetic NCAM mimetic, the P2 peptide. Like NCAM itself, the peptide both triggered neurite extension and a [Ca2+]i rise in primary neurons. The amplitude of the observed [Ca2+]i responses to P2d was dependent on how long neurons had been grown in culture, being about threefold higher in cultures grown for 7 d than in cultures grown for only 6 h. Moreover, at identical times in vitro, an amplitude of P2d-induced [Ca2+]i rise was significantly higher in the neurons isolated from the P5 animals than in the neurons isolated from the E19 animals, suggesting a developmental change of Ca2+ responses to the peptide. This difference might be caused by changing levels of NCAM/FGFR expression, or more likely, by an age-dependent increase in the density of L-type VDCCs (Porter et al., 1997), which together with T-type channels, are the main factors responsible for depolarization-induced Ca2+ influx into the somata of hippocampal neurons. In accordance with this, the P2d-induced [Ca2+]i could be significantly inhibited by blockers of both L- and T-type but not N-type VDCCs (summarized in Figure 9). Because N- and P/Q-type VDCCs are sparsely distributed on the neuronal somata but abundant in growth cones (Catterall, 2000), it cannot be ruled out that these channels may mediate P2d-induced Ca2+ entry into the nerve terminals. However, in P2d-triggered neurite outgrowth they seemed to play a minor role.
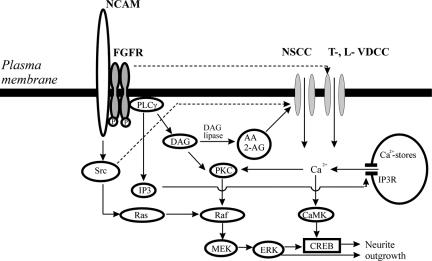
Figure 9.
Schematic representation of signaling pathways activated by P2d interaction with NCAM. CaMK, calcium-calmodulin kinase; CREB, cAMP response element-binding protein; ERK, extracellular signal-regulated kinase; PKC, protein kinase C; MEK, mitogen-activated protein kinase kinase; Src, Src-family kinases. See Discussion for details.
Upstream of the VDCCs, the P2d-mediated signaling involved RTKs; PLCγ1, an enzyme predominantly associated with RTKs; and DAG-lipase (Figure 9). This is in agreement with observations by Saffell et al. (1997) and Archer et al. (1999) that homophilic CAM binding sequentially induces phosphorylation of FGFR, activation of PLCγ, and generation of DAG. The major Ca2+ channels type affected by DAG and/or its products has previously been suggested to be the L-type (Archer et al., 1999), presumably activated either by a direct interaction with FGFR2 (Rosenthal et al., 2001) or indirectly by the DAG metabolite AA (Archer et al., 1999) and/or by the AA-activated cannabinoid receptor 1 (Williams et al., 2003). However, to our knowledge, no evidence of direct activation by AA of voltage-gated Ca2+ channels at near-basal membrane potentials exists. Moreover, AA has been shown to reduce L-type Ca2+ current in cardiac cells (Petit-Jacques and Hartzell, 1996), rat sympathetic neurons (Liu and Rittenhouse, 2000), and rat ventricular myocytes (Xiao et al., 1997). No effect of AA on the basal L-current density has been observed in cardiac myocytes (Petit-Jacques and Hartzell, 1996), and basic fibroblast growth factor (FGF), the high-affinity ligand of FGFR, does not affect activation or inactivation kinetics of L-current at the near-basal potentials (Rosenthal, et al., 2001). Thus, given the significant depolarization required for opening of either L- or N-type Ca2+ channels, it seems unlikely that AA activates them directly. Instead, 2-AG or AA might first activate their well-known effectors, the NSCCs. In agreement with this hypothesis, the P2d-induced [Ca2+]i rise was only partially inhibited by blockers of L-type VDCCs. However, both T-type VDCCs and NSCCs were important for induction of Ca2+ responses by the NCAM mimetic. Thus, NSCCs might be the channels, which open first in response to P2d and mediate an “initial” Ca2+ influx and depolarization of the plasma membrane. This then leads to the opening of low-voltage–activated T-type Ca2+ channels, which mediate further depolarization, resulting in an opening of high-voltage–activated L-type VDCCs and in an increase of Ca2+ entry. In addition, a direct activation of L-type of VDCCs by FGFR2, which also is expressed in cultured hippocampal neurons (Neiiendam et al., 2004), might contribute to the induced Ca2+ influx (Rosenthal et al., 2001; Figure 9, stippled arrow).
The NSCCs types mediating the P2d-induced Ca2+ entry might be arachidonate-regulated Ca2+ channels and/or the transient receptor potential (TRP) family Ca2+-permeable channels, both activated by 2-AG, AA, and its metabolites (reviewed in Hardie, 2003; Shuttleworth and Mignen, 2003). Recently, it has been shown that the TRPC1 channels of the TRP family colocalize with FGFR1 and contribute to the FGFR1-mediated Ca2+ influx in embryonic rat neural stem cells by an unknown mechanism (Pla et al., 2005). Because TRPC1 can heterotetramerize with other TRPCs expressed during brain developments (e.g., with TRPC3/TRPC6, TRPC4/TRPC5; Strubing et al., 2003), it is possible that FGFR1 coexists in complexes with TRPCs in primary neurons. In this case, activation of FGFR1 would result in a local increase of DAG and its products, leading to an efficient activation of the adjacent TRP channels.
In our experiments, the P2d-elicited [Ca2+]i rise was only partially inhibited by blockage of the FGFR1–PLC γ–DAG signaling pathway but abolished by an RTK/protein tyrosine kinase blocker lavendustin A. This can be explained by an “additional” activation of L-type VDCCs by FGFR2 (see Discussion above) and/or by Src-family kinases. Indeed, both P2d-induced neurite outgrowth and Ca2+ responses were significantly inhibited by blocking of Src. Moreover, treatment with P2d resulted in the phosphorylation of the Src-family kinases on Tyr416, an event known to mediate Src activation, and the time course of the phosphorylation correlated to that of P2d-induced Ca2+ responses. This is in accordance with previous reports demonstrating that Src kinases are phosphorylated by homophilic NCAM binding (Beggs et al., 1997) or by another synthetic peptide ligand of NCAM, C3d (our unpublished observations). Tyrosine phosphorylation of Src kinases can positively regulate NSCCs (Hisatsune et al., 2004; Vazquez et al., 2004) and L-type VDCC (Hou et al., 2003, and references therein), thus explaining the dependence of P2d-elicited Ca2+ responses on the activity of Src kinases (Figure 9, stippled arrow).
Activation of the Src-associated signaling cascade may not be universally required for the cellular effects exerted by NCAM. However, this cascade might be of importance under circumstances where the FGFR-associated signaling is not “sufficient” to induce a given cellular response. In particular, in cultures of embryonic hippocampal neurons, neurite extension cannot be elicited by an activation of FGFR with its high-affinity ligand FGF2 (Amoureux et al., 2000). However, FGF2 has been demonstrated to induce neurite outgrowth from postnatal dorsal root ganglion and cerebellar neurons (Archer et al., 1999; Neiiendam et al., 2004), thus exemplifying principal differences in the outputs of FGFR activation in the above-mentioned experimental systems.
Importantly, the Ca2+-regulating mechanisms shown to be involved in the P2d-induced intracellular signaling (Src-family kinases, T-types VDCCs, and NSCCs) are also crucial for NCAM-induced differentiation of primary neurons. However, our data suggest that T-type VDCCs and NSCCs are not only involved in the NCAM-induced neurite outgrowth but also may participate in the induction of neurite extension induced by other factors.
In our experiments, we found that the relative contribution of FGFR- and Src-mediated signaling pathways in P2d-versus NCAM-induced responses differed. The reason might be that homophilic NCAM binding is mediated by interactions between several NCAM modules, namely, the IgI and IgII modules mediate dimerization of NCAM molecules situated on the same cell surface, whereas the IgI and IgIII, IgII and IgII, and the IgII and IgIII modules of NCAM mediate trans homophilic binding (Soroka et al., 2003). The P2d-peptide selectively mimicks only the IgI to IgII binding. Therefore, the P2d- and NCAM-induced responses probably result in a different dynamics of NCAM clustering and thus in different contribution of of Src (Fyn) phosphorylation known to be induced by this clustering (Beggs et al., 1997).
In conclusion, we have identified two signaling pathways associated with FGFR and Src-family kinases, respectively, mediating the intracellular Ca2+ signaling induced by the NCAM mimetic P2d. Nonselective cation conductances and T-type VDCCs, but not L-type VDCCs, were crucial for the induction of P2d-mediated cellular responses. Moreover, Src-family kinases, T-type VDCCs, and NSCCs all were important for NCAM-induced neurite extension. This suggests that multiple signaling pathways and mechanisms of Ca2+ homeostasis contribute to NCAM-triggered neuronal differentiation depending on cell type and developmental stage.
Acknowledgments
This work has been supported by Danish Medical Research Council, Lundbeck Foundation, European Union integrated project PROMEMORIA, and Danish Cancer Society.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–10–0987) on March 1, 2006.
Abbreviations used: 2-AG, 2-arachidonoyl glycerol; AA, arachidonic acid; DAG, diacylglycerol; GAP, growth-associated protein; NSCC, nonselective cation channel; PLC, phospholipase C; RTK, receptor tyrosine kinase; TRP, transient receptor potential; VDCC, voltage-dependent Ca2+ channel.
References
- Amoureux, M., Cunningham, B., Edelman, G., and Crossin, K. (2000). N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype. J. Neurosci. 20, 3631–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer, F., Doherty, P., Collins, D., and Bolsover, S. (1999). CAMs and FGF cause a local submembrane calcium signal promoting axon outgrowth without a rise in bulk calcium concentration. Eur. J. Neurosci. 11, 3565–3573. [DOI] [PubMed] [Google Scholar]
- Beggs, H., Baragona, S., Hemperly, J., and Maness, P. (1997). NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn). J. Biol. Chem. 272, 8310–8319. [DOI] [PubMed] [Google Scholar]
- Catterall, W. (2000). Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555. [DOI] [PubMed] [Google Scholar]
- Doherty, P., Ashton, S., Moore, S., Doherty, P., Ashton, S., Moore, S., and Walsh, F. (1991). Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell 67, 21–33. [DOI] [PubMed] [Google Scholar]
- Hardie, R. (2003). Regulation of TRP channels via lipid second messengers. Annu. Rev. Physiol. 65, 735–759. [DOI] [PubMed] [Google Scholar]
- Hinsby, A., Berezin, V., and Bock, E. (2004). Molecular mechanisms of NCAM function. Front. Biosci. 9, 2227–2244. [DOI] [PubMed] [Google Scholar]
- Hisatsune, C., Kuroda, Y., Nakamura, K., Inoue, T., Nakamura, T., Michikawa, T., Mizutani, A., and Mikoshiba, K. (2004). Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 279, 18887–18894. [DOI] [PubMed] [Google Scholar]
- Hou, X., Zhang, G., Yan, J., and Liu, Y. (2003). Increased tyrosine phosphorylation of alpha(1C) subunits of L-type voltage-gated calcium channels and interactions among Src/Fyn, PSD-95 and alpha(1C) in rat hippocampus after transient brain ischemia. Brain Res. 979, 43–50. [DOI] [PubMed] [Google Scholar]
- Irving, A., and Collingridge, G. (1998). A characterization of muscarinic receptor-mediated intracellular Ca2+-mobilization in cultured rat hippocampal neurons. J. Physiol. 511, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Kim, J., Zilberstein, A., Margolis, B., Kim, J., Schlessinger, J., and Rhee, S. (1991). Platelet-derived growth factor stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell 65, 435–441. [DOI] [PubMed] [Google Scholar]
- Kiryushko, D., Berezin, V., and Bock, E. (2004). Regulators of neurite outgrowth, role of cell adhesion molecules. Ann. NY Acad. Sci. 1014, 140–154. [DOI] [PubMed] [Google Scholar]
- Kiryushko, D., Novitskaya, V., Soroka, V., Klingelhofer, J., Lukanidin, E., Berezin, V., and Bock, E. (2006). Molecular mechanisms of Ca2+ signaling in neurons induced by the S100A4 protein. Mol. Cell. Biol. (in press). [DOI] [PMC free article] [PubMed]
- Kiselyov, V., Soroka, V., Berezin, V., and Bock, E. (2005). Structural biology of NCAM homophilic binding and activation of FGFR. J. Neurochem. 94, 1169–1179. [DOI] [PubMed] [Google Scholar]
- Kolkova, K., Novitskaya, V., Pedersen, N., Berezin, V., and Bock, E. (2000). NCAM-stimulated neurite outgrowth depends on activation of protein kinase C (PKC) and the Ras-mitogen activation protein kinase pathway. J. Neurosci. 20, 2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Kolkova, K., Rudenko, O., Soroka, V., Poulsen, F., Bock, E., and Berezin, V. (2005). Triple effect of mimetic peptides interfering with neural cell adhesion molecule homophilic cis interactions. Biochemistry 44, 5034–5040. [DOI] [PubMed] [Google Scholar]
- Liu, L., and Rittenhouse, A. (2000). Effects of arachidonic acid on unitary calcium currents in rat sympathetic neurons. J. Physiol. 2, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maar, T., Rønn, L., Bock, E., Berezin, V., Moran, J., Pasantes-Morales, H., and Schousboe, A. (1997). Characterization of microwell cultures of dissociated brain tissue for studies of cell-cell interactions. J. Neurosci. Res. 47, 163–172. [PubMed] [Google Scholar]
- Neiiendam, J., Kohler, L., Christensen, C., Li, S., Pedersen, M., Ditlevsen, D., Kornum, M., Kiselyov, V., Berezin, V., and Bock, E. (2004). An NCAM-derived FGF-receptor agonist, the FGL-peptide, induces neurite outgrowth and neuronal survival in primary rat neurons. J. Neurochem. 91, 920–935. [DOI] [PubMed] [Google Scholar]
- Nilius, B., and Droogmans, G. (2003). Amazing chloride channels: an overview. Acta Physiol. Scand. 177, 119–147. [DOI] [PubMed] [Google Scholar]
- Petit-Jacques, J., and Hartzell, H. (1996). Effect of arachidonic acid on the L-type calcium current in frog cardiac myocytes. J. Physiol. 15, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla, A., Maric, D., Brazer, S., Giacobini, P., Liu, X., Chang, Y., Ambudkar, I., and Barker, J. (2005). Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 25, 2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, N., Thibault, O., Thibault, V., Chen, K.-C., and Landfield, P. (1997). Calcium channel density and hippocampal cell death with age in long-term culture. J. Neurosci. 17, 5629–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlsen, G., Ditlevsen, D., Berezin, V., and Bock, E. (2003). (2003). Intracellular signaling by the neural cell adhesion molecule. Neurochem. Res. 28, 127–141. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R., Thieme, H., and Strauss, O. (2001). Fibroblast growth factor receptor 2 (FGFR2) in brain neurons and retinal pigment epithelial cells act via stimulation of neuroendocrine L-type channels (Ca(v)1.3). FASEB J. 15, 970–977. [DOI] [PubMed] [Google Scholar]
- Rønn, L., Dissing, S., Holm, A., Berezin, V., and Bock, E. (2002). Increased intracellular calcium is required for neurite outgrowth induced by a synthetic peptide ligand of NCAM. FEBS Lett. 518, 60–66. [DOI] [PubMed] [Google Scholar]
- Saffell, J., Williams, E., Mason, I., Walsh, F., and Doherty, P. (1997). Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron 18, 231–242. [DOI] [PubMed] [Google Scholar]
- Schuch, U., Lohse, M., and Schachner, M. (1989). Neural cell adhesion molecules influence second messenger systems. Neuron 3, 13–20. [DOI] [PubMed] [Google Scholar]
- Shuttleworth, T., and Mignen, O. (2003). Calcium entry and the control of calcium oscillations. Biochem. Soc. Trans. 31, 916–919. [DOI] [PubMed] [Google Scholar]
- Soroka, V., Kiryushko, D., Novitskaya, V., Ronn, L., Poulsen, F., Holm, A., Bock, E., snf Berezin, V. (2002). Induction of neuronal differentiation by a peptide corresponding to the homophilic binding site of the second Ig module of the neural cell adhesion molecule. J. Biol. Chem. 277, 24676–24683. [DOI] [PubMed] [Google Scholar]
- Soroka, V., et al. (2003). Structure and interactions of NCAM Ig1-2-3 suggest a novel zipper mechanism for homophilic adhesion. Structure 11, 1291–1301. [DOI] [PubMed] [Google Scholar]
- Stein, V., Hermans-Borgmeyer, I., Jentsch, T., and Hubner, C. (2004). Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 468, 57–64. [DOI] [PubMed] [Google Scholar]
- Strubing, C., Krapivinsky, G., Krapivinsky, L., and Clapham, D. (2003). Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 278, 39014–39019. [DOI] [PubMed] [Google Scholar]
- Vazquez, G., Wedel, B., Kawasaki, B., Bird, G., and Putney, J., Jr. (2004). Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J. Biol. Chem. 279, 40521–40528. [DOI] [PubMed] [Google Scholar]
- Von Bohlen Und Halback, F., Taylor, J., and Schachner, M. (1992). Cell type-specific effects of the neural adhesion molecules L1 and N-CAM on diverse second messenger systems. Eur. J. Neurosci. 4, 896–909. [DOI] [PubMed] [Google Scholar]
- Walmod, P., Kolkova, K., Berezin, V., and Bock, E. (2004). Zippers make signals, NCAM-mediated molecular interactions and signal transduction. Neurochem. Res. 29, 2015–2035. [DOI] [PubMed] [Google Scholar]
- Williams, E., Walsh, F., and Doherty, P. (2003). The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 160, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y., Gomez, A., Morgan, J., Lederer, W., and Leaf, A. (1997). Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. USA 94, 4182–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]