Abstract
Objectives. This study sought to verify the independent role of heart rate in the prediction of all-cause, cardiovascular, and noncardiovascular mortality in a low-risk male population.
Methods. In an Italian population-based observational study, heart rate was measured in 2533 men, aged 40 to 69 years, between 1984 and 1993. Data on cardiovascular risk factors were collected according to standardized procedures. Vital status was updated to December 1997.
Results. Of 2533 men followed up (representing 24 457 person-years), 393 men died. Age-adjusted death rates for 5 heart rate levels showed increasing trends. The adjusted hazard rate ratios for each heart rate increment were 1.52 (95% confidence interval [CI] = 1.29, 1.78) for all-cause mortality, 1.63 (95% CI = 1.26, 2.10) for cardiovascular mortality, and 1.47 (95% CI = 1.19, 1.80) for noncardiovascular mortality. Relative risks between extreme levels were more than 2-fold for all endpoints considered.
Conclusions. Heart rate is an independent predictor of cardiovascular, noncardiovascular, and total mortality in this Italian middle-aged male population.
A direct association of elevated heart rate with hypertension, hypercholesterolemia, and hyperglycemia and an inverse relationship with physical activity and pulmonary function have been found in several epidemiologic studies.1–11 Tachycardia precedes hypertension, increases risk of arrhythmia, and facilitates the development of atherosclerotic plaque.8,9,12
All-cause mortality seems to be higher in individuals with elevated heart rates. In the Framingham Study, death rates increased with increasing levels of heart rate; the association was stronger in men and not related to age, and there was no indication of a threshold value that could be defined as hazardous.13,14 Among individuals with hypertension, a positive association between heart rate and cardiovascular diseases, particularly coronary heart disease and sudden death, has also been shown even after exclusion of early deaths, confirming that heart rate is an independent predictor of fatal events and not only an indicator of preexisting illness.15 The National Health and Nutrition Examination Survey, the Paris Prospective Study, the Spandau Health Test, and the 3 Chicago epidemiologic studies have also revealed a positive association between heart rate and cardiovascular and noncardiovascular mortality.6,7,16–20 These findings represent evidence that heart rate can be considered a determinant of health status and a predictor of poor outcomes in the general population.
Despite the amount of international evidence, mainly from Northern European and US studies, that heart rate is a health determinant, the prognostic role of heart rate in mortality has not been assessed in Mediterranean populations. Among these groups, other risk factors, such as dietary intake, serum cholesterol, and body mass index, have been shown to have unique relations to total mortality and fatal coronary events.21–23 In the present study, we evaluated the association of heart rate with all-cause, cardiovascular, and noncardiovascular mortality among a sample of middle-aged men residing in central Italy.
METHODS
Between 1984 and 1993, the Malattie Cardiovascolari Aterosclerotiche, Istituto Superiore di Sanità (MATISS) Project screened 2533 men aged 40 to 69 years who resided in 4 municipalities of central Italy approximately 100 km southeast of Rome (Figure 1 ▶). Participants represented 58% of a random sample, stratified by age and sex, selected from the electoral rolls. Data on cardiovascular risk factors were collected according to procedures and methodologies described in detail elsewhere.24
FIGURE 1—
Map of Italy delineating the area covered by the MATISS Project.
Resting electrocardiograms (ECGs) were read according to the Minnesota code (a standard ECG coding procedure that provides a framework for uniform reporting in homogeneously and precisely defined classes). Heart rate was calculated as mean number of beats per minute.
Total serum cholesterol (mg/dL) was centrally tested by the Laboratory of Clinical Biochemistry, Istituto Superiore di Sanità, via an automated enzymatic colorimetric method (Boehringer Mannheim GmBH Diagnostica, Monza, Italy). Preciset cholesterol solutions (Boehringer Mannheim GmBH Diagnostica, Monza, Italy) were used as calibrators, and a calibration curve was elaborated for each run. As a means of providing internal quality control, commercial controls were analyzed in each run.
During the screening period, the laboratory performing serum lipid level analyses was assessed in regard to quality control by the World Health Organization Lipid Reference Center in Prague. For each control sample, bias was estimated as the difference between the observed and reference values and expressed as a percentage of the reference value. The average bias, calculated on the basis of 18 different sets of samples received between February 1984 and December 1995, was –1.1% (range: –5.9% to 2.6%). Local hospitals assessed fasting blood glucose (mg/dL), using an automated enzymatic method.
Blood pressure (mm Hg) was measured with subjects in a sitting position after a 4-minute rest. Measurements were taken in the right arm with a standard mercury sphygmomanometer. Two consecutive readings were recorded, and their mean was used for the analysis.
A wall tape and a lever balance were used to measure height and weight with subjects in light underwear. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Arm circumference was measured on the right arm at mid-distance from the acromion to the olecranon. The contribution of skin and subcutaneous tissue, estimated via triceps skinfold thickness, was removed through the use of a simple formula: rough circumference – (skinfold × π). As suggested in previous analyses,10,25 this variable was used as the best available indicator of muscle mass and, indirectly, one of the possible indicators of physical fitness.
Two spirometric curves were recorded, and the vital capacity and 1-second forced expiratory volume (mL/m2) of the technically best curve were tabulated. Each value was then divided by the corresponding squared height to adjust pulmonary function for individual body size.
Smoking habits and number of cigarettes smoked per day were recorded. Diagnosis of diabetes at baseline was based on the presence of a fasting blood glucose level of 120 mg/dL or higher or a specific treatment.
Diagnosis of cardiovascular diseases included cases of myocardial infarction and cerebrovascular diseases; diagnostic criteria for myocardial infarction included personal history and ECG Minnesota codes, in accord with Seven Countries Study procedures.26 Diagnostic criteria for cerebrovascular disease included only personal history collected via a standard questionnaire. Sixty-three subjects with a history of cardiovascular disease were not excluded from subsequent analyses, but presence of such a history has been evaluated as a possible risk factor for subsequent fatal events.
The examined population was followed up for 4 to 13 years (until December 1997) in regard to vital status, migration, and mortality (total number of person-years: 24 457). Twenty subjects who emigrated contributed to the study until they relocated. There were no other losses to follow-up.
The International Classification of Diseases, Ninth Revision (ICD-9) was used in coding causes of death. Death certificates were not validated but were coded according to National Institute of Statistics procedures that provide standard coding priorities (violence, cancer, myocardial infarction, stroke, others). In cases involving uncertainty, subjects' personal physicians were asked to provide further information.
Age-adjusted mean values of continuous variables and proportions of categorical variables were computed in 5 heart rate classes (<60, 60–69, 70–79, 80–89, and ≥90 beats per minute). Differences across classes were evaluated through one-way analyses of variance, Pearson χ2 tests, and simple and multiple linear regression analyses with heart rate as the dependent variable.
Age-standardized all-cause (ICD-9 codes 001–999), cardiovascular (ICD-9 codes 390–459), and noncardiovascular (all causes of death other than cardiovascular) mortality rates were computed for the 5 heart rate classes. Increasing or decreasing linear trends across levels were assessed with Cochran statistical tests.27
The Cox proportional hazards model comprises 3 different multivariate analyses and uses procedures available in the BMDP2L statistical program.28 All-cause mortality, cardiovascular mortality, and noncardiovascular mortality were dependent variables, while heart rate, age, systolic blood pressure, serum cholesterol level, number of cigarettes smoked per day, BMI, arm circumference, adjusted forced expiratory volume in 1 second, diabetes, and preexisting cardiovascular disease were independent variables. Multivariate coefficients for heart rate as a continuous variable were used to describe mortality risk curves (all causes, cardiovascular, and noncardiovascular) as a function of increasing heart rate values.
We ran the same models, treating heart rate as a categorical variable and including 4 dummy variables for the 5 heart rate classes. The proportionality assumption requires that the ratio of hazard rates for different levels of an independent variable be constant. In this case, the proportionality assumption for heart rate was tested and confirmed for the 5 classes. The z2 test29 produced a nonsignificant value of 1.42. Possible interactions between heart rate and other factors were tested in all models. Univariate analyses were carried out for the entire sample (n = 2533). Multivariate analyses included 2233 subjects (88%) for whom risk factor measurements were available and complete.
RESULTS
In total, 2533 men were screened and followed up (representing 24 457 person-years); 393 deaths were recorded (38% due to cardiovascular diseases, 39% due to cancer, 5% due to violence, 14% due to other causes of death, and 4% due to unknown causes). Table 1 ▶ reports numbers of deaths and person-years by heart rate categories and outcomes.
TABLE 1—
Person-Years and Number of Deaths, by Heart Rate Class and Outcome: MATISS Project, 1984–1997
| Cause of Death | ||||
| Heart Rate, Beats per Minute | Person-Years | All Causes, No. | Cardiovascular, No. | Noncardiovascular, No. |
| < 60 | 7 271 | 86 | 31 | 55 |
| 60–69 | 9 337 | 119 | 47 | 72 |
| 70–79 | 5 041 | 105 | 38 | 67 |
| 80–89 | 1 825 | 42 | 19 | 23 |
| ≥ 90 | 983 | 36 | 13 | 23 |
| Total | 24 457 | 388 | 148 | 240 |
Systolic blood pressure, number of cigarettes per day, BMI, and prevalence of diabetes showed increases with increasing heart rate, and forced expiratory volume decreased with increasing heart rate (Table 2 ▶). Table 3 ▶ shows that all-cause and cardiovascular (but not noncardiovascular) death rates regularly increased from the lowest to the highest heart rate levels. Relative risks between extreme classes were always more than 2-fold, and trends were statistically significant for all-cause, cardiovascular, and noncardiovascular mortality.
TABLE 2—
Characteristics of Study Population, by Heart Rate Class: MATISS Project, 1984–1997
| Heart Rate, Beats per Minute | |||||||
| < 60 (n = 642) | 60–69 (n = 836) | 70–79 (n = 477) | 80–89 (n = 181) | ≥ 90 (n = 97) | F | P | |
| Systolic blood pressure, mm Hg, mean ± SD | 140.3 ± 20.0 | 141.5 ± 19.6 | 143.5 ± 20.1 | 148.4 ± 23.9 | 150.5 ± 25.8 | 10.07 | .000 |
| No. of cigarettes smoked per day, mean ± SD | 6.6 ± 10.1 | 7.6 ± 10.4 | 8.0 ± 10.9 | 8.6 ± 12.1 | 7.9 ± 10.6 | 1.97 | .096 |
| Body mass index, kg/m2, mean ± SD | 27.3 ± 3.6 | 27.3 ± 3.7 | 27.6 ± 3.6 | 27.2 ± 3.8 | 28.0 ± 4.1 | 1.40 | .232 |
| Serum cholesterol level, mg/dL, mean ± SD | 219.9 ± 40.3 | 222.5 ± 42.4 | 224.3 ± 43.8 | 233.1 ± 48.5 | 229.3 ± 44.9 | 4.01 | .003 |
| Forced expiratory volume, mL/m2, mean ± SD | 953 ± 190 | 943 ± 189 | 913 ± 185 | 855 ± 220 | 876 ± 187 | 13.14 | .000 |
| Arm circumference, cm, mean ± SD | 27.3 ± 2.9 | 27.3 ± 2.8 | 27.2 ± 3.0 | 27.2 ± 2.7 | 27.2 ± 2.8 | 0.15 | .962 |
| Prevalence of diabetes, % | 4.3 | 3.4 | 7.1 | 7.8 | 9.3 | 15.94a | .003 |
aPearson χ2.
TABLE 3—
Age-Standardized Yearly Death Rates (× 100 000), by Heart Rate Class: MATISS Project, 1984–1997
| All Causes | Cardiovascular | Noncardiovascular | |||||
| Heart Rate, Beats per Minute | No. Exposed | Rate | SE | Rate | SE | Rate | SE |
| < 60 | 733 | 1259 | 124 | 455 | 80 | 804 | 103 |
| 60–69 | 938 | 1405 | 111 | 587 | 77 | 818 | 90 |
| 70–79 | 546 | 2042 | 167 | 755 | 116 | 1287 | 145 |
| 80–89 | 200 | 2086 | 288 | 947 | 214 | 1139 | 231 |
| ≥ 90 | 116 | 3001 | 400 | 1044 | 281 | 1957 | 352 |
| Test for trend, P | .0000 | .0004 | .0000 | ||||
| Relative risk between extreme classes (95% confidence interval) | 2.38 (1.71, 3.36) | 2.29 (1.22, 4.32) | 2.43 (1.59, 3.82) | ||||
Table 4 ▶ shows that men with heart rates of 90 beats per minute or higher, relative to men with heart rates below 60 beats per minute, were at increased risk of all-cause mortality (hazard rate ratio = 2.67, 95% confidence interval [CI] = 1.76, 4.04), cardiovascular mortality (hazard rate ratio = 2.54, 95% CI = 1.25, 5.16), and noncardiovascular mortality (hazard rate ratio = 2.87, 95% CI = 1.71, 4.79).
TABLE 4—
Hazard Rate Ratios, by Cause of Death and Heart Rate Class: MATISS Project, 1984–1997
| Cause and Heart Rate, Beats per Minute | No. of Deaths | Multivariate Hazard Rate Ratiob | 95% Confidence Interval |
| All causes | (n = 350)a | ||
| < 60 | 78 | Reference | |
| 60–69 | 113 | 1.16 | 0.87, 1.55 |
| 70–79 | 90 | 1.61 | 1.18, 2.20 |
| 80–89 | 37 | 1.38 | 0.93, 2.06 |
| ≥ 90 | 32 | 2.67 | 1.76, 4.04 |
| Cardiovascular | (n = 133)a | ||
| < 60 | 27 | Reference | |
| 60–69 | 44 | 1.38 | 0.85, 2.24 |
| 70–79 | 34 | 1.87 | 1.11, 3.16 |
| 80–89 | 17 | 1.78 | 0.95, 3.34 |
| ≥ 90 | 11 | 2.54 | 1.25, 5.16 |
| Noncardiovascular | (n = 217)a | ||
| < 60 | 51 | Reference | |
| 60–69 | 69 | 1.06 | 0.74, 1.53 |
| 70–79 | 56 | 1.50 | 1.02, 2.20 |
| 80–89 | 20 | 1.17 | 0.69, 1.98 |
| ≥ 90 | 21 | 2.87 | 1.71, 4.79 |
aN = 2232.
bAdjusted for age, systolic blood pressure, serum cholesterol, cigarettes smoked per day, body mass index, arm circumference, adjusted forced expiratory volume, diabetes, and preexisting cardiovascular disease.
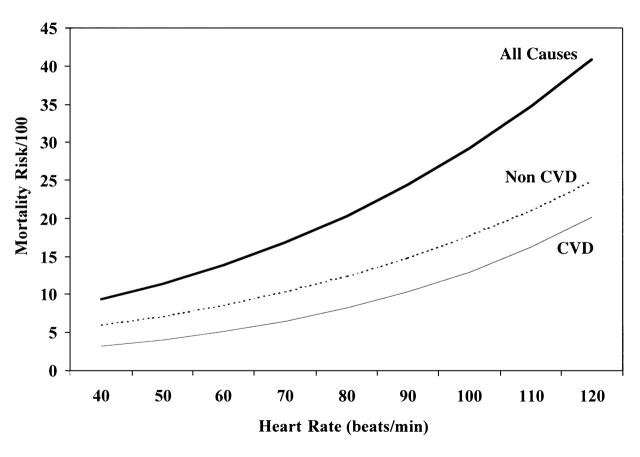
Multivariate heart rate coefficients were 0.0209 (t2221 = 5.13) for all-cause mortality, 0.243 (t2221 = 3.75) for cardiovascular disease mortality, and 0.0192 (t2221 = 3.65) for noncardiovascular mortality (Figure 2 ▶). Corresponding multivariate hazard rate ratios computed for each heart rate increment of 20 beats per minute were 1.52 (95% CI = 1.29, 1.78), 1.63 (95% CI = 1.26, 2.10), and 1.47 (95% CI = 1.19, 1.80).
FIGURE 2—
All-cause, cardiovascular disease (CVD), and noncardiovascular (Non CVD) mortality risks as a function of heart rate levels among men aged 40–69 years: MATISS Project, 1984–1997.
DISCUSSION
Like other researchers,2–6 we observed a direct association of heart rate with systolic blood pressure, number of cigarettes smoked per day, serum cholesterol level, and diabetes; an inverse relationship with pulmonary function; and no association with age. Although an association between heart rate and cardiovascular disease has not always been found,13,14,30–32 there is general agreement that heart rate is a strong determinant of overall mortality.6,7,16–20,33
In the present study, multivariate analyses of all-cause, cardiovascular, and noncardiovascular mortality showed an increasing trend when moving from the lowest to the highest heart rate levels; relative risks between extreme classes were 2.7 for all-cause mortality, 2.5 for cardiovascular mortality, and 2.9 for noncardiovascular mortality. Trends were adjusted for age, systolic blood pressure, number of cigarettes smoked per day, BMI, arm circumference, adjusted forced expiratory volume, diabetes, and preexisting cardiovascular disease. For the 3 endpoints, the risk functions obtained by applying the multivariate coefficients to arbitrary heart rate levels confirmed that death risks increase continuously when moving from the lowest to the highest heart rate levels, as suggested in previous studies.13,14 There was no indication of a threshold value that could be defined as hazardous.
Moreover, all-cause mortality exhibited almost the same sharp increase, confirming the role of heart rate as a general indicator of health status.12,34 To this extent, it can be stated that in the present study, the lack of death certificate validation did not diminish the significance of the results, because our objective was more to evaluate the role of heart rate as a predictor of health status than to establish its etiologic role in the prediction of each different cause of death.
Physical activity is one of the most important mechanisms thought to explain the association between heart rate and health status.13,33 This information was not available in the MATISS Project. Arm circumference, together with measurements of pulmonary function, can be used as an indicator of physical fitness in the absence of physical activity data. Adjustment for these variables did not reduce the association.
Heart rate exhibits great intraindividual variability; is influenced by infections, recent physical activity, and anxiety and stress35–37; and is related to intrinsic pulse, a personal characteristic that reflects an individual's ability to support exertion, and, together with other factors, contributes to assessments of physical fitness.38 Moreover, intraindividual fluctuations can partly be a result of daily variations and measurement errors related to the environmental conditions under which ECGs are recorded (e.g., resting time before measurement, room temperature, and time of day).1 For all of these reasons, many authors suggest that caution be exercised in treating heart rate as an independent predictor of mortality.1 Heart rate has been related to all-cause mortality and specific causes in many studies, however, and in spite of different methodological approaches.
Resting heart rate is a marker of hemodynamic and autonomic nervous system states that can cause elevations in cardiovascular mortality by inducing atherosclerosis and producing rhythm disturbances.2 A chronic predominance of sympathetic over parasympathetic activity, via either beta-adrenergic stimulation or alpha-adrenergic vasoconstriction, can cause insulin resistance, which, in turn, is involved in the genesis of hypertension, hypercholesterolemia, and hyperglycemia.1,12,39
Tachycardia per se increases cardiac work and represents a marker of increased sheer force in large vessels that, independently of blood pressure, can be conducive to high arterial rigidity and thus atherosclerosis.40,41 Although heart rate is an important predictor of hypertension in normotensive and borderline subjects and is a risk factor for death, the role of tachycardia is generally underestimated in clinical practice. Among hypertensive individuals, the possible presence of tachycardia is often ignored or considered as an indicator of benign conditions or transient anxiety. The beneficial effects of beta-blockers on survival revealed in some studies could be partly associated with their ability to lower heart rate.42
Heart rate can be considered an important indicator of mortality among the Italian male population. It represents one of the most important independent predictors of cardiovascular, noncardiovascular, and overall mortality in that, other risk factors being equal, death risks increase about 50% for each 20-beat-per-minute increment, and relative risks between extreme heart rate levels are more than 2-fold. Moreover, heart rate is relatively easy to record and can be modified, thus making it useful in terms of primary and secondary prevention.
Further studies, particularly natural experiments describing the predictive role of heart rate changes over time (either spontaneous or induced by intervention), are necessary. At present, knowledge regarding the link between heart rate and mortality suggests that control of heart rate (i.e., by promotion of physical activity and healthy lifestyles) can improve life expectancy in a general middle-aged male population. In addition, until more intervention data are available, the relation of heart rate to other recognized risk factors underlines the validity of using high heart rates as risk markers for more vigorous interventions involving standard risk factors.
Acknowledgments
This research was supported by “Il Progetto CUORE—Epidemiology and Prevention of Ischaemic Heart Disease,” an Italian project financed by the Ministry of Health. Informed consent was obtained from all MATISS Project participants.
We thank the staff of the MATISS Project, in particular Augusto Santaquilani, Piergiorgio Zuccaro, Patrizia Caiola De Sanctis, and Agata Poce.
F. Seccareccia analyzed the data and wrote the paper. F. Pannozzo was responsible for follow-up of the data. F. Dima read and coded the electrocardiograms. A. Minoprio and A. Menditto carried out the laboratory tests for blood lipids. C. Lo Noce was responsible for the fieldwork. S. Giampaoli supervised the data analysis and contributed to the writing of the paper.
Peer Reviewed
References
- 1.Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15:3–17. [DOI] [PubMed] [Google Scholar]
- 2.Palatini P, Julius S. Association of tachycardia with morbidity and mortality: pathophysiological considerations. J Hum Hypertens. 1997;11(suppl 1):S19–S27. [PubMed] [Google Scholar]
- 3.Palatini P, Casiglia E, Pauletto P, Staessen J, Kaciroti N, Julius S. Relationship of tachycardia with high blood pressure and metabolic abnormalities: a study with mixture analysis in three populations. Hypertension. 1997;30:1267–1273. [DOI] [PubMed] [Google Scholar]
- 4.Wannamethee G, Shaper G. The association between heart rate and blood pressure, blood lipids and other cardiovascular risk factors. J Cardiovasc Risk. 1994;1:223–230. [DOI] [PubMed] [Google Scholar]
- 5.Gillum RF. The epidemiology of resting heart rate in a national sample of men and women: associations with hypertension, coronary heart disease, blood pressure, and other cardiovascular risk factors. Am Heart J. 1988;116:163–174. [DOI] [PubMed] [Google Scholar]
- 6.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. [DOI] [PubMed] [Google Scholar]
- 7.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. [DOI] [PubMed] [Google Scholar]
- 8.Blair SN, Goodyear NN, Gibbons LW, Copper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252:487–490. [PubMed] [Google Scholar]
- 9.Paffenbarger RS Jr, Jung DL, Leung RW, Hyde RT. Physical activity and hypertension: an epidemiological review. Ann Intern Med. 1991;23:319–327. [DOI] [PubMed] [Google Scholar]
- 10.Seccareccia F, Menotti A. Physical activity, physical fitness and mortality in a sample of middle-aged men followed-up 25 years. J Sports Med Phys Fitness. 1992;32:206–213. [PubMed] [Google Scholar]
- 11.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. [DOI] [PubMed] [Google Scholar]
- 12.Julius S, Palatini P, Nesbitt SD. Tachycardia: an important determinant of coronary risk in hypertension. J Hypertens Suppl. 1998;16:S9–S15. [PubMed] [Google Scholar]
- 13.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J. 1985;109:876–885. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 15.Gillman MW, Kannel WB, Belanger A, D'Agostino R. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. [DOI] [PubMed] [Google Scholar]
- 16.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I epidemiologic follow-up study. Am Heart J. 1991;121:172–177. [DOI] [PubMed] [Google Scholar]
- 17.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. [DOI] [PubMed] [Google Scholar]
- 18.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population. Hypertension. 1999;33:44–52. [DOI] [PubMed] [Google Scholar]
- 19.Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18:1404–1410. [DOI] [PubMed] [Google Scholar]
- 20.Greenland P, Daviglus ML, Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–862. [DOI] [PubMed] [Google Scholar]
- 21.Seccareccia F, Lanti M, Menotti A, Scanga M, and the RIFLE Research Group. Role of body mass index in the prediction of all cause mortality in over 62,000 men and women. The Italian RIFLE Pooling Project. J Epidemiol Community Health. 1998;52:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spagnolo A, Menotti A, Giampaoli S, et al. High density lipoprotein cholesterol distribution and predictive power in some Italian population studies. Eur J Epidemiol. 1989;5:328–335. [DOI] [PubMed] [Google Scholar]
- 23.Farchi G, Fidanza F, Mariotti S, Menotti A. Is diet an independent risk factor for mortality? 20 year mortality in the Italian rural cohorts of the Seven Countries Study. Eur J Clin Nutr. 1994;48:19–29. [PubMed] [Google Scholar]
- 24.Rose G, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. 2nd ed. Geneva, Switzerland: World Health Organization; 1982.
- 25.Menotti A, Mariotti S, Seccareccia F, Giampaoli S. The 25 year estimated probability of death from some specific causes as a function of twelve risk factors in middle-aged men. Eur J Epidemiol. 1988;4:60–67. [DOI] [PubMed] [Google Scholar]
- 26.Keys A. Coronary Heart Disease in Seven Countries. New York, NY: American Heart Association; 1970.
- 27.Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–441. [Google Scholar]
- 28.Dixon WJ. BMDP Statistical Software Manual. Berkeley: University of California Press; 1992.
- 29.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons Inc; 1980.
- 30.Tibblin G, Wilhelmsen L, Werko L. Risk factors for myocardial infarction and death due to ischemic heart disease and other causes. Am J Cardiol. 1975;35:514–522. [DOI] [PubMed] [Google Scholar]
- 31.Keys A, Taylor HL, Blackburn H. Mortality and coronary heart disease among men studied for 23 years. Arch Intern Med. 1971;128:201–214. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RJ, Burchfield CM, Benfante R, Chiu D, Reed DM, Yano K. Lifestyle and biological factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch Intern Med. 1995;155:686–694. [PubMed] [Google Scholar]
- 33.Wannamethee G, Shaper G, Macfarlane PW. Heart rate, physical activity and mortality from cancer and other noncardiovascular diseases. Am J Epidemiol. 1993;137:735–748. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RJ, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women. The Framingham Study. Arch Intern Med. 1996;156:505–509. [PubMed] [Google Scholar]
- 35.Johnston D, Anastasiades P. The relationship between heart rate and mood in real life. J Psychosom Res. 1990;34:21–27. [DOI] [PubMed] [Google Scholar]
- 36.Miller SB. Parasympathetic nervous system control of heart rate responses to stress in offspring of hypertensives. Psychophysiology. 1994;31:11–16. [DOI] [PubMed] [Google Scholar]
- 37.Benschop RJ, Nieuwenhuis EES, Tromp EAM, Godaert GLR, Ballieux RE, Van Doornen LJP. Effects of beta-adrenergic blockade on immunologic and cardiovascular changes induced by mental stress. Circulation. 1994;89:762–769. [DOI] [PubMed] [Google Scholar]
- 38.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125:726–731. [DOI] [PubMed] [Google Scholar]
- 39.Pyorala K, Savolainen E, Kaukola S, Haapakoski J. Plasma insulin as a coronary heart disease risk factor: relationship to other risk factors and predictive value during 9 1/2 year follow-up of the Helsinki Policemen Study population. Acta Med Scand Suppl. 1985;701:38–52. [DOI] [PubMed] [Google Scholar]
- 40.Sa Cunha R, Pannier B, Benetos A, et al. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertens. 1997;15:1423–1430. [DOI] [PubMed] [Google Scholar]
- 41.Gordon D, Guyton I, Karnovsky N. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest. 1983;45:14–19. [PubMed] [Google Scholar]
- 42.Kjeshus JK. Comments on beta-blockers: heart rate reduction, a mechanism of action. Eur Heart J. 1985;6:29–30. [Google Scholar]