Abstract
Objectives. This study modeled the health and federal fiscal effects of expanding Medicaid for HIV-infected people to improve access to highly active antiretroviral therapy.
Methods. A disease state model of the US HIV epidemic, with and without Medicaid expansion, was used. Eligibility required a CD4 cell count less than 500/mm3 or viral load greater than 10 000, absent or inadequate medication insurance, and annual income less than $10 000. Two benefits were modeled, “full” and “limited” (medications, outpatient care). Federal spending for Medicaid, Medicare, AIDS Drug Assistance Program, Supplemental Security Income, and Social Security Disability Insurance were assessed.
Results. An estimated 38 000 individuals would enroll in a Medicaid HIV expansion. Over 5 years, expansion would prevent an estimated 13 000 AIDS diagnoses and 2600 deaths and add 5816 years of life. Net federal costs for all programs are $739 million (full benefits) and $480 million (limited benefits); for Medicaid alone, the costs are $1.43 and $1.17 billion, respectively. Results were sensitive to awareness of serostatus, highly active antiretroviral therapy cost, and participation rate. Strategies for federal cost neutrality include Medicaid HIV drug price reductions as low as 9% and private insurance buy-ins.
Conclusions. Expansion of the Medicaid eligibility to increase access to antiretroviral therapy would have substantial health benefits at affordable costs.
Antiretroviral therapy dramatically slows the progression of HIV disease and AIDS. Multiple clinical studies have reported that triple therapy with 2 nucleoside analogues and a protease inhibitor or nonnucleoside reverse transcriptase inhibitor—known as highly active antiretroviral therapy—sharply depresses viral load, improves CD4 cell counts, and delays clinical progression to AIDS and death.1–4
Although there is now hope that HIV/AIDS can be a manageable chronic disease,5,6 clinical efficacy has not been matched by access to and use of therapy. Approximately 750 000 individuals in the United States are infected with HIV,7 most of whom likely meet the broad criteria for being offered highly active antiretroviral therapy (CD4 cell count < 500/mm3, viral load > 10 000 HIV RNA copies/mL, or symptoms). Yet, evidence from clinical settings suggests that highly active antiretroviral therapy is used by only about 200 000 individuals.8 Reasons for limited use include lack of awareness of infection, physician failure to initiate treatment, provider or patient preference to postpone treatment, patient difficulty with adherence or uncomfortable side effects, and development of drug resistance.6
One critical barrier is financial. Many individuals lack adequate medical insurance or the financial resources to afford the $12 000 or more annual cost of highly active antiretroviral therapy,9–13 and competing financial demands are associated with reduced antiretroviral use.14 Several public programs attempt to improve financial access to care. Medicaid, with funding shared by the federal government and the states, is the largest payer of health care for persons with HIV/AIDS, accounting for $3.9 billion in fiscal year 1999 and covering 46% of all patients with HIV in care.15,16 Medicaid eligibility requires individuals to have low income and to match an eligibility category. Most persons with HIV/AIDS qualify for Medicaid by meeting the disability and income criteria of the federal Supplemental Security Income cash assistance program for persons who are aged, blind, or disabled. However, people in the early stages of HIV disease, for whom highly active antiretroviral therapy may be indicated and may prevent disability, face the catch-22 of having eligibility postponed until they become disabled.
Other public programs increase access to HIV medications for those with lower incomes. The most important is the AIDS Drug Assistance Program (ADAP) of the federal Ryan White CARE Act. Unlike Medicaid, which is an entitlement program, the Ryan White Act requires annual appropriations; fiscal year 1998 funding for ADAP was $510 million, about two-thirds federal and one-third state. State ADAPs have faced repeated funding crises because of increasing demands from current and prospective enrollees.17 ADAP has 2 significant limitations: adequate program funding to meet rising need depends each year on political support in Congress, and ADAP pays almost exclusively for prescription medications, not the full range of health care required by people with HIV.
One approach to lessen financial barriers to earlier HIV care and highly active antiretroviral therapy is expanding Medicaid eligibility for individuals with early HIV disease, before disability.18 However, federal policy requires that, absent legislated changes, modifications to eligibility or benefits be budget neutral to Medicaid: total program costs cannot exceed those expected without a modification. Preliminary federal analyses concluded that for an HIV expansion, budget neutrality for Medicaid was unlikely, stalling efforts to expand eligibility for HIV.19
We undertook our analysis to assess more comprehensively the likely health and federal fiscal effects of a Medicaid eligibility expansion for people with HIV disease. Specifically, we designed a model to quantify an expansion's effect on the use of highly active antiretroviral therapy and consequently on AIDS diagnoses, deaths, and years of life. We estimated federal fiscal effects for a range of federal programs: Medicaid and 4 other health and income support programs that support significant numbers of persons with HIV/AIDS. We also examined several strategies to reduce or eliminate net federal costs and net costs to Medicaid over a 5-year time frame.
METHODS
We developed a computer spreadsheet model that incorporates epidemiologic, clinical, and insurance data to estimate health outcomes and costs initially and over time for an expansion of Medicaid eligibility to persons with HIV infection. In this section, we summarize the model's components and outcomes, the value of key input parameters, and characteristics of the modeled expansion. A detailed technical report is available from the authors.
Model Components and Outcomes
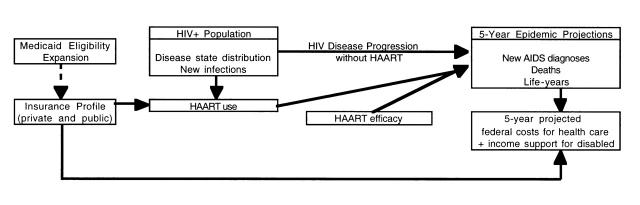
The core of our analysis was a disease state–transition (Markov) model of HIV disease progression (Figure 1 ▶). This model portrays how the mix of HIV disease in the US population evolves over a period of 5 years. It is based on estimates of HIV disease progression with limited (no, single, or dual drug) antiretroviral therapy as well as the added clinical benefit of highly active antiretroviral therapy. Increased insurance coverage, such as with a Medicaid expansion, slows disease progression by increasing the likelihood of highly active antiretroviral therapy use.
FIGURE 1—
Structure of model of the health and federal fiscal impacts of expanding Medicaid for people with HIV to improve access to highly active antiretroviral therapy (HAART).
We calculated 3 health outcomes.
New AIDS diagnoses represent the progression from any pre-AIDS state to AIDS (by the Center for Disease Control and Prevention's 1993 definition).20
Deaths include all causes, as generally reported.
Life-years are cumulative years of life for all individuals who are HIV infected, unadjusted for quality of life.
Use of highly active antiretroviral therapy slows the progression through disease states, thereby improving these health outcomes.
The model also calculated 2 fiscal outcomes: federal costs represent costs for Medicaid, Medicare, ADAP, Supplemental Security Income, and Social Security Disability Insurance. We concentrated on federal costs because state Medicaid 1115 expansion waivers require federal cost neutrality. To assess strategies to reach federal cost neutrality, we varied factors such as Medicaid drug price reductions and expansion eligibility and examined drug revenues with higher sales volume and prices low enough for neutrality.
The health and fiscal effects of the Medicaid HIV expansion were calculated by comparing outcomes of the 5-year simulation (1998–2003) with and without the expansion. Because of the lack of definitive empirical data for many inputs and also uncertainty about program design, we conducted sensitivity analyses on all inputs.
Value of Key Input Parameters
Epidemiologic and clinical inputs are described in Table 1 ▶, with sources. We estimated from published sources an initial HIV-infected US population of 750 000 (250 000 with AIDS and 500 000 with HIV disease pre-AIDS), with two thirds aware of their HIV status and 200 000 receiving highly active antiretroviral therapy. HIV disease progression rates were derived from 30 natural history studies, with updating of summary estimates to reflect current treatment and mortality patterns. Highly active antiretroviral therapy efficacy in reducing progression was estimated from 22 clinical trials, classified by stage of HIV disease and types of therapy compared.
TABLE 1—
HIV Epidemic, Treatment, and Disease Progression Inputs
| Disease Progression Inputs | |||||
| HIV/AIDS Disease State | Size of Population; Proportion Aware of HIV Infectiona | Proportion Receiving HAARTb | Target States | Transition Probability per Quarter Without HAARTc | Reduction in Transition With HAARTd |
| 0. Uninfectede | 250 × 106 | . . . | 1 | 0.00004 | . . . |
| 1. Asymptomatic, CD4 > 500 | 167 000; 0.40 | 0.047 | 2 | 0.02 | 0.91 |
| 3 | 0.01 | 0.91 | |||
| 4 | 0.002 | 0.91 | |||
| 5 | 0.0005 | 0.91 | |||
| 6 | 0.0018 | 0.84 | |||
| 2. Asymptomatic, CD4 = 201–500 | 167 000; 0.40 | 0.096 | 3 | 0.04 | 0.9 |
| 4 | 0.005 | 0.9 | |||
| 5 | 0.003 | 0.9 | |||
| 6 | 0.0027 | 0.82 | |||
| 3. Symptomatic, pre-AIDS | 167 000; 0.75 | 0.18 | 4 | 0.06 | 0.87 |
| 5 | 0.02 | 0.87 | |||
| 6 | 0.003 | 0.79 | |||
| 4. AIDS, 1993 definitionf | 150 000; 0.85 | 0.48 | 5 | 0.03 | 0.54 |
| 6 | 0.012 | 0.7 | |||
| 5. AIDS, 1987 definition | 100 000; 1.0 | 0.79 | 6 | 0.047 | 0.67 |
| 6. Death | 0; . . . | . . . | . . . | . . . | . . . |
Note. HAART = highly active antiretroviral therapy.
aSources. Karon and Rosenberg7; Holmberg21; Rosenberg22; HIV/AIDS Surveillance Report23; Sweeney et al.24
bProportion receiving HAART is for all those who are HIV infected; the denominator includes those unaware of infection or not in care. The total is 200 000. Ellipses indicate data that were not applicable. Sources. Palella et al.4; Gruta et al.8; Moorman et al.25
cTransition probabilities without HAART assume current mix of non-HAART use; see text. Sources. Cohort studies,26–55 adjusted for current mono- and dual-antiretroviral use.4
dReductions in transition with HAART reflect clinical trials2–4,56–74; differences within pre-AIDS stages and within AIDS stages reflect a different mix of non-HAART antiretroviral therapy.4
eStage 0 transition yields HIV incidence in the United States of 40 000 per year.
fStage 4 (AIDS by the 1993 definition) excludes AIDS by the 1987 definition and includes CD4 ≤ 200. The model includes a stage “4 incident” to which prior states progress, to adjust for the use of prevalent cohorts to calibrate progression from stage 4. The progression rate from 4 incident to 4 is 0.1200 per quarter, and the decrease with HAART is 0.54.
The medication payer distribution, by HIV stage of disease, is shown in Table 2 ▶. Medication payers include Medicaid, private, ADAP, safety net (e.g., charity care), and none.
TABLE 2—
Distribution of Medication Payer for Each HIV Disease State
| Medication Payera | |||||
| Disease State | Medicaidb | Private | ADAP | Safety Net | None |
| 1 | 0.220 | 0.405 | 0.045 | 0.035 | 0.295 |
| 2 | 0.240 | 0.400 | 0.045 | 0.050 | 0.265 |
| 3 | 0.270 | 0.360 | 0.060 | 0.080 | 0.230 |
| 4 | 0.350 | 0.265 | 0.135 | 0.120 | 0.130 |
| 5 | 0.440 | 0.250 | 0.175 | 0.060 | 0.075 |
Note. ADAP = AIDS Drug Assistance Program.
Source. Data from the HIV Costs and Service Utilization Study (J. Fleishman, PhD, written communication, November 18, 1997); the National Alliance of State and Territorial AIDS Directors/AIDS Drug Assistance Program (J. Kelly, written communication, June 3, 1998); and Foster et al.75
aEach row totals to 1.0.
bThe Medicaid category excludes those with inadequate medication coverage; these individuals appear in other columns.
We estimated the annual cost of medical care without highly active antiretroviral therapy at $4829 in early-stage HIV and $31 308 in late-stage AIDS, based on pre-1996 studies of populations insured mainly by Medicaid, adjusted for recent trends in inpatient and outpatient utilization.4,76–80 This is consistent with findings from the national HIV Cost and Services Utilization Study of $20 000 per year across disease stages.15 The cost with highly active antiretroviral therapy is $15 404 in early-stage HIV and $30 261 in late-stage AIDS. This reflects the cost of highly active antiretroviral therapy ($12 310 average wholesale price for indinavir, zidovudine, and lamivudine; 20% less for Medicaid) and adjustments for utilization changes seen with highly active antiretroviral therapy (e.g., 55% reduction in inpatient care)12,80–82 (also J. G. Kahn, MD, MPH, University of California, San Francisco, unpublished data, 1999).
Characteristics of the Modeled Expansion
Eligibility for the modeled Medicaid HIV expansion requires that individuals meet 3 conditions: (1) satisfy clinical guidelines for offering highly active antiretroviral therapy: CD4 cell count less than 500/mm3, viral load greater than 10 000 HIV RNA copies/mL, or HIV symptoms6; (2) lack medical insurance for antiretroviral medications, having no means to pay for medications, or relying on safety net programs; and (3) earn less than $10 000 per year (124% of the federal poverty level for a single person).83
We estimated enrollment as follows. Clinical eligibility was assumed to be 100% during AIDS and 77% pre-AIDS.26,84,85 Inadequate medical insurance (ADAP, safety net, or none) was 31% to 39% by HIV stage (Table 2 ▶). We assumed no decrease in private insurance (“crowd-out”) as a result of the expansion. Income eligibility was 55% to 57%, derived from data on income distribution by payer (J. Fleishman, written communication, November 18, 1997; J. Kelly, written communication, June 3, 1998) extended to distribution by disease stage. Among eligible individuals, enrollment varied by HIV stage (asymptomatic 60%, symptomatic 75%, and clinical AIDS 20% because these individuals failed to exercise disability-associated Medicaid eligibility), informed by experience from non-HIV insurance programs86,87 (also C. B. Livingston, written communication, October, 1998). We assumed highly active antiretroviral therapy use by 90% of the expansion enrollees.
We modeled 2 benefit packages. The “full” benefit package covered all Medicaid inpatient, outpatient, and home health services and medications. The “limited” Medicaid benefit package covered only outpatient services (including mental health and substance abuse counseling covered under Medicaid) and medications. We assumed equal rates of enrollment, use of highly active antiretroviral therapy, and slowing of disease progression for the 2 packages.
RESULTS
Eligibility Expansion Enrollees
The model predicted that initially 38 000 persons would be enrolled in the expansion. Forty-five percent (17 200) would have had no prior access to highly active antiretroviral therapy (no medication payer); the remainder (20 800) would have had limited access via ADAP and other safety net payers. The expansion enrollees would have annual incomes distributed across 3 categories ($0: 11%; $1–$5000: 45%; and $5001–$10 000: 44%). Most enrollees (70%) would be pre-AIDS, with 60% symptomatic and 10% asymptomatic; 30% would have AIDS. Enrollment in the expansion is projected to increase to 51 000 at the end of 5 years. This growth reflects both new enrollment (because of increasing HIV prevalence and also severity—persons with early HIV disease sicken and thus are more likely to be eligible and enroll) and high program retention (stabilization of HIV disease with highly active antiretroviral therapy prevents progression to Medicaid-qualifying disability).
Health Outcomes
The projected health outcomes are presented in Table 3 ▶. The model predicts 49 745 new AIDS diagnoses in the first year in the absence of an eligibility expansion and 46 313 with expanded Medicaid access. New AIDS diagnoses would decline in subsequent years for both scenarios as the pre-AIDS population decreases: individuals who develop AIDS or die are incompletely replaced by people with very early HIV disease or new infection. The Medicaid HIV expansion would lead to a decrease of 13 108 in the total number of new AIDS diagnoses over 5 years. The annual difference would decrease (from 3432 in year 1 to 1978 in year 5) because slowed disease progression with more highly active antiretroviral therapy use would increase the number of individuals at risk for progressing to AIDS compared with the current insurance mix. With the analysis extended to 10 years, the estimated total decrease in new AIDS diagnoses is 20 507.
TABLE 3—
Health Outcomes (New AIDS Diagnoses, Deaths, and Life-Years) and Fiscal Outcomes for the Current Insurance Mix, With the Medicaid HIV Eligibility Expansion Implemented, and the Difference, by Year
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total 5 Years | Total 10 Years | |
| New AIDS diagnoses | |||||||
| Current insurance mix | 49 745 | 44 535 | 40 451 | 37 294 | 34 893 | 206 918 | 355 351 |
| Medicaid expansion | 46 313 | 41 599 | 37 912 | 35 070 | 32 916 | 193 810 | 334 844 |
| Difference | –3432 | –2935 | –2539 | –2225 | –1978 | –13 108 | –20 507 |
| Deaths | |||||||
| Current insurance mix | 18 209 | 18 872 | 19 659 | 20 479 | 21 280 | 98 497 | 208 892 |
| Medicaid expansion | 17 763 | 18 390 | 19 134 | 19 909 | 20 665 | 95 862 | 202 798 |
| Difference | –446 | –481 | –524 | –570 | –615 | –2635 | –6094 |
| Life-years | |||||||
| Current insurance mix | 758 215 | 779 768 | 800 595 | 820 611 | 839 806 | 3 998 995 | 8 460 145 |
| Medicaid expansion | 758 380 | 780 390 | 801 715 | 822 272 | 842 053 | 4 004 811 | 8 487 464 |
| Difference | 165 | 623 | 1120 | 1661 | 2248 | 5816 | 27 319 |
| Federal spending, $ billion | |||||||
| Current insurance mix | 4.15 | 4.58 | 4.94 | 5.26 | 5.53 | 24.46 | 55.39 |
| Full benefit | 4.36 | 4.76 | 5.09 | 5.37 | 5.62 | 25.20 | 56.14 |
| Difference | 0.21 | 0.18 | 0.15 | 0.11 | 0.09 | 0.74 | 0.75 |
| Limited benefit | 4.31 | 4.71 | 5.04 | 5.32 | 5.56 | 24.94 | 55.55 |
| Difference | 0.16 | 0.13 | 0.10 | 0.06 | 0.03 | 0.48 | 0.16 |
The model predicts about 18 200 deaths per year. For both current insurance and the expansion, total deaths per year would rise slightly over 5 years. In the first year, about 450 deaths would be averted by implementing the expansion; over 5 years, more than 2600 deaths would be averted. For 10 years, the estimated total decrease in deaths is 6094.
Life-years for all individuals who are HIV infected are estimated to total 3.999 million over 5 years with current insurance and 4.005 million with the eligibility expansion. The annual amount rises slowly, because slightly fewer people die than are newly infected. The increase in life-years associated with implementing the expansion would be only 165 in the first year, because many of the 446 deaths would be averted late in the year (the model portrays the year in quarters). The annual difference in life-years would grow with the increase in cumulative deaths averted, reaching 2248 in year 5. The total difference in life-years would be 5816 over the 5 years of the analysis. Over 10 years, the estimated total difference in life-years is 27 319.
Fiscal Outcomes
Expected federal costs.
The model predicts that total federal spending on HIV/AIDS in the 5 programs we model will be $24.5 billion over 5 years without an eligibility expansion (Table 3 ▶). This includes $10 billion for Medicaid, $6 billion for Medicare, and smaller amounts for ADAP, Supplemental Security Income, and Social Security Disability Insurance.
With an eligibility expansion, the “full” Medicaid benefit package would have a net federal cost to the 5 programs of $739 million over 5 years. Net Medicaid-only spending would increase by $1.43 billion. Spending would decrease for all other programs: ADAP by $410 million (because some individuals shift from ADAP to Medicaid), Medicare by $100 million, and Supplemental Security Income and Social Security Disability Insurance by $180 million (both because of slowing of disease progression). The “limited” Medicaid benefit package would have a net federal cost of $480 million. As with the full benefit package, net Medicaid-only spending would increase sharply ($1.17 billion) over 5 years, partially offset by decreases in other programs. Extended to a 10-year time frame, the limited benefit would have a net cost of $166 million, as savings in Medicaid and other programs resulting from slowed disease progression accumulate more rapidly than do costs. The full benefit would have a net cost of $750 million over 10 years, similar to results for 5 years.
Federal cost neutrality.
There are multiple potential strategies to reduce costs and thus move toward federal cost neutrality for the 5 programs and for Medicaid alone. In this section, we discuss strategies that require changes in existing Medicaid practice: discounts for HIV drugs and payment of private health insurance premiums. Under sensitivity analyses, we examine how the design of the expansion itself may facilitate budget neutrality (e.g., with stricter clinical or income eligibility criteria).
For both benefit package options, budget neutrality can be achieved with an additional reduction in drug prices paid by Medicaid for beneficiaries who are HIV infected (regardless of eligibility category). For the 5 federal programs, a 14.5% reduction in drug prices generates zero net costs with the full benefit package, and a 9.4% reduction yields zero net costs with limited benefits. Medicaid-only budget neutrality can be reached with HIV drug price reductions of 28% and 23%, respectively.
Budget neutrality also might be reached if a significant portion of HIV-related medical care under the expansion were obtained through the purchase of community-rated health insurance (i.e., by paying premiums for COBRA [Consolidated Omnibus Budget Reconciliation Act] to continue employment-based health coverage or for state high-risk pools). For example, the full benefit package would be budget neutral for the 5 federal programs if 45% of all expansion participants, spread across disease states, received care via the payment of annual premiums of $2400 instead of actual medical costs. The limited benefit would require 36% participation in such a program to achieve budget neutrality. The corresponding COBRA or insurance purchasing participation rate for Medicaid-only neutrality is high: 87% for the full or limited benefit package.
Because cost neutrality is very sensitive to drug price reductions, we estimated the net revenue effect of an expansion accompanied by HIV drug price reductions adequate for budget neutrality. We assumed that increases in drug use are reflected in sales volume. We estimated that a 9.4% reduction in price (required to achieve budget neutrality with the limited benefit package for the 5-program analysis) would result in a drug revenue decrease of about $25 million over 5 years. This means that the proportionate increase in sales volume almost equals the proportionate reduction in price. If the drug price reduction strategy is combined with limited payment of private insurance premiums, then net drug revenues increase: with 10% participation in COBRA or risk pools, the required drug price reduction for budget neutrality is 6.8%, and net drug revenues rise by $229 million over 5 years.
With the full benefit package, the 14.5% reduction required to achieve budget neutrality leads to a decrease in drug revenues of about $514 million. COBRA or risk pool participation of 17% or higher results in a net drug revenue increase; at 20%, the increase is $108 million. For the Medicaid-only analysis, drug revenues decrease by $1.8 billion and $1.3 billion for the full and limited benefit packages, respectively, if neutrality is based solely on lower drug prices. COBRA or risk pool participation rates of 58% and 52%, respectively, keep drug revenues stable.
Sensitivity Analyses
Selected sensitivity analyses for fiscal effect on the 5 federal programs analysis are presented in Table 4 ▶, organized by category of model input. For each input, we defined a plausible range of values and then reported the net costs associated with this range for the limited and full benefit packages. We report results only for inputs with uncertainty that affects predicted net federal fiscal costs by $150 million or more (full sensitivity analyses are available from the authors).
TABLE 4—
Selected Sensitivity Analysesa: Expected 5-Year Net Federal Costs for Range in Values of Key Model Inputs
| Net Cost Over 5 Years, $ Million | |||
| Range in Input Valueb | Limited Benefit Package | Full Benefit Package | |
| Base case | . . . | 480 | 739 |
| Population characteristics | |||
| Distribution between pre-AIDS and AIDS: proportion in pre-AIDS disease states | 0.8–1.2 | 535–407c | 812–648c |
| Awareness of serostatus overall | 0.8–1.2 | 375–574 | 579–873 |
| Clinical factors | |||
| Rate of disease progression overall | 0.8–1.2 | 567–394c | 819–659c |
| Rate of progression to AIDS | 0.8–1.2 | 577–389c | 831–652c |
| HAART efficacy overall | 0.5–1.2d | 698–397c | 939–664c |
| HAART efficacy symptomatic pre-AIDS | 0.5–1.2 | 697–398c | 947–661c |
| Costs of HIV/AIDS medical care | |||
| Cost of HAART | 0.8–1.2 | 300–659 | 559–918 |
| Cost of HIV medical care, excluding drugs | 0.6–1.4 | 418–541 | 574–904 |
| Insurance mix | |||
| % With no medication payer | 0.6–1.4 | 383–574 | 619–857 |
| Eligibility expansion | |||
| Participation rate | 0.4–1.3 | 198–592 | 303–914 |
| Exclusion of AIDS | . . . | 137 | 264 |
| Inclusion of $10 000–$17 000 income group | . . . | 668 | 1036 |
Note. HAART = highly active antiretroviral therapy. Ellipses indicate that no input range is needed—as is standard for the base case and for sensitivity analyses that are categoric rather than quantitative (e.g., complete exclusion of a category of patients).
aResults are reported only for inputs with uncertainty that affects predicted net federal fiscal costs by $150 million or more; full sensitivity analyses are available from the authors.
bValues indicate proportion of base case.
cInverse relation between input and outcome values; i.e., higher input value yields lower net cost.
dBecause maximum HAART efficacy is 100%, high base case estimates of efficacy (e.g., 90%) were increased by <20% for the scenario assessing 1.2 times base case.
The inputs that most affected predicted net costs for the 5 programs were awareness of serostatus (range in net costs = $200–$300 million), efficacy of highly active antiretroviral therapy (range = about $300 million), costs of highly active antiretroviral therapy (range = about $350 million), percentage with no medication insurance (range = about $200 million), expansion participation rate (range = $400–$600 million), exclusion of individuals with AIDS (range = $350–$500 million), and likelihood of meeting income rules and inclusion of the $10 000 to $17 000 income group (range = $200–$300 million).
Of note, several inputs made relatively little difference in predicted net costs. These include population characteristics and the mix of medication payers (range = $25–$150 million) and rate of progression to death (range = $20 million) (not reported in Table 4 ▶). Also, there was only moderate sensitivity to the overall cost of HIV/AIDS medical care (excluding medications) within the limited benefit analysis (range = $120 million, Table 4 ▶). This is because higher nonmedication health care costs have counteracting effects: they increase the cost for expansion enrollees but also increase Medicaid and other program savings.
We also conducted sensitivity analyses for Medicaid-only net costs. The inputs that most affected predicted net costs were awareness of serostatus (range in net costs = $500–$600 million), the cost of highly active antiretroviral therapy (range = $350 million), the cost of other medical care (range = $200–$400 million), the expansion participation rate (range = $1–$1.2 billion), the likelihood of meeting income rules (range = $300–$400 million), inclusion of the $10 000 to $17 000 income group (range = $400–$600 million), and exclusion of individuals with AIDS (range = $500–$700 million). Medicaid net cost was relatively unaffected by highly active antiretroviral therapy efficacy because savings to Supplemental Security Income, Social Security Disability Insurance, and Medicare were not counted.
We performed a sensitivity analysis to represent use of multiple-drug “salvage” antiretroviral therapy for a small portion of those receiving highly active antiretroviral therapy. Salvage therapy, commonly defined as 4 to 6 or more antiretroviral drugs, is used when 3-drug highly active antiretroviral therapy fails clinically or virologically.88 We assumed that 5% of all individuals receiving highly active antiretroviral therapy would receive 6 drugs, at twice the cost of 3-drug highly active antiretroviral therapy, and that efficacy with highly active antiretroviral therapy would increase by the same absolute 5%. We found that clinical benefits rose almost 10% (e.g., AIDS diagnoses prevented increase from 13 100 to 14 260). Five-program net federal costs rose slightly (for the full benefit, from $739 to $759 million; for the limited benefit, from $480 to $498 million). Medicaid-only net costs also rose slightly (for the full benefit, from $1.43 to $1.47 billion; for the limited benefit, from $1.17 to $1.22 billion).
DISCUSSION
This analysis suggests that substantial health benefits are likely to accrue from expanding access to antiretroviral treatment, such as a hypothetical change in Medicaid eligibility modeled here. We estimated that if 38 000 persons with HIV had expanded access to highly active antiretroviral therapy, it would result in a decrease over 5 years of more than 13 000 new AIDS diagnoses and 2600 deaths, leading to an increase of more than 5800 years of life. The net federal cost of the expansion was estimated at $96 to $148 million per year over 5 years, when Medicaid and 4 other federal health and income support programs were considered. This cost could be offset by 9% to 15% decreases in the prices of HIV drugs for Medicaid and other cost-saving strategies. The net federal cost of the expansion for Medicaid alone was estimated at $234 to $286 million per year (0.1% of total federal Medicaid spending and 23%–29% of HIV spending).
The results of the analysis were particularly sensitive to the size of the eligible population (e.g., to the number of people who knew they were infected and who met eligibility criteria) and to the likelihood that eligible individuals enroll. The results were also quite sensitive to the cost and, for some analyses, efficacy of antiretroviral therapy with protease inhibitors.
Our results are consistent with published cost-effectiveness analyses of highly active antiretroviral therapy. These analyses suggested costs per quality-adjusted life-years of about $15 000 for therapy initiated earlier in the course of HIV disease.89–91 When we used our model to evaluate highly active antiretroviral therapy rather than the Medicaid expansion, we estimated the cost per life-year gained at $11 000. To perform this calculation, we used the 10-year time frame (most life-years are gained after 5 years), assumed that all expansion enrollees lacked prior access to highly active antiretroviral therapy (in the reported analysis, some obtained highly active antiretroviral therapy through programs such as ADAP), limited intervention costs to antiretroviral drugs and incremental outpatient care, and tracked all medical costs regardless of payer. In contrast, in the Medicaid expansion analysis, the federal cost per added year of life was generally higher (up to $120 000) and was very sensitive to the particular programs tracked. The higher calculated cost per life-year reflects the shorter time frame, prior use of highly active antiretroviral therapy by many enrollees, and nondrug expansion costs such as inpatient care.
This study has several important limitations. Perhaps foremost, some input data had a weak empirical basis. We conducted extensive sensitivity analyses, and it was reassuring that many factors appeared to have little effect on results within plausible uncertainty ranges.
Second, we did not model the clinical benefits of being in regular medical care other than that associated with highly active antiretroviral therapy (e.g., prophylaxis against opportunistic infections). This is a conservative bias because such effects would increase health benefits and decrease costs.
Third, we did not model changes in the value of key inputs over time. For example, we assumed that long-term clinical efficacy of highly active antiretroviral therapy was equal to initial efficacy. Another example is the cost of highly active antiretroviral medications, which may decrease as competition increases and pharmaceutical companies recoup development costs or may increase with the availability of new agents.
Fourth, we did not portray HIV infections averted by the suppression of infectivity by highly active antiretroviral therapy, a phenomenon suggested by sharply lowered viral loads but as yet unconfirmed.
Fifth, we did not include possible savings in outpatient management of opportunistic infections (i.e., highly active antiretroviral therapy lowering the need for chronic therapy),92 although we did assume lower inpatient use for opportunistic infections. We also did not model the costs of monitoring of CD4 cell count and viral load to determine who is eligible for this program. This annual cost of several hundred dollars per person ineligible for the expansion likely would be funded through non-Medicaid mechanisms, such as local health budgets.
Sixth, we modeled 250 000 persons with AIDS in 1998. An updated value of 300 000 would raise net costs, because care for enrollees with AIDS is more expensive. Finally, we modeled expansion eligibility based on now-outdated clinical guidelines recommending antiretroviral therapy at a CD4 count less than 500/mm3. Using the guidelines revision from early 2001 (including CD4 < 350/mm3), our model estimated net 5-year federal costs of $581 million (21% lower than our base case) and added life-years of 5252 (8% lower).
Our analysis differed in 2 ways from the current federal approach to Medicaid eligibility expansions. First, we examined 5 federal programs. Under current policy, proposed eligibility expansions are evaluated from the fiscal perspective of Medicaid alone. We took this academic license to consider medical and income support programs that we thought reasonable to include in an accounting of federal budget effects. Even so, our analysis may be conservative because we omitted economic effects such as increases in tax revenues resulting from delayed disability. If each averted case of AIDS disability adds 2 years of productivity, federal revenues rise considerably. Second, the limited benefit package is a departure from traditional Medicaid expansions and waivers. There may be policy concerns about the feasibility and fairness of partial benefits and hence a reluctance to broadly pursue this strategy.
Another issue is that we portrayed a national Medicaid expansion. Although there have been congressional bills proposing expansion (in 2000, H.R. 1591 and S. 902), federal policy change is far from assured. Expansion in the near term is more likely to be initiated by state waiver proposals to the Centers for Medicare and Medicaid Services. Maine, Massachusetts, and the District of Columbia recently received such waivers. State-level analyses of health and fiscal effects must reflect local conditions such as epidemic size, use of highly active antiretroviral therapy, Medicaid eligibility and benefits, other programs to support highly active antiretroviral therapy access, and the specific expansion features. A state-level analysis also could examine fiscal effects for state and local payers. Depending on the federal match, state Medicaid costs might be similar to federal costs, but state and local savings could be substantially enhanced by reduced indigent care costs.
Techniques to reach federal budget neutrality will have important fiscal consequences for key actors in HIV care. For example, using Medicaid funds to purchase private insurance translates to private insurers and subscribers subsidizing HIV care. This subsidy could conceivably destabilize small or marginal risk pools. Reductions in payments for inpatient or outpatient care would decrease income to Medicaid providers, who are mostly public and already underfunded. This could threaten their viability and reduce access for Medicaid enrollees. Drug price reductions would decrease revenues to drug companies, as we quantified. However, the decision by Glaxo Wellcome to lower the developing world price of zidovudine by 75%93 suggests that moderate drug price reductions to facilitate reaching resource-poor populations may be possible. There is also a history of discounts for US public sector health programs, including Medicaid drug pricing and larger discounts on drugs and vaccines for the US Public Health Service. The Medicaid HIV expansion waiver for Maine is based on the receipt of a drug price discount for waiver participants.
Another complicated sphere is the interaction of Medicaid and private insurance. In a phenomenon known as crowd-out, some individuals with private insurance (or their employers) take advantage of broadened public insurance eligibility, increasing public costs without increasing coverage. Crowd-out is a less serious problem with the limited benefit and may be minimized by excluding individuals with private coverage in the prior 90 days. A related issue is identifying when public funding is required to pay individual COBRA premiums, perhaps necessitating special financial eligibility evaluations.
Despite the challenges of implementing a Medicaid HIV expansion, we believe it is an important strategy for improving access to highly active antiretroviral therapy. It eliminates the current eligibility catch-22 faced by many people living with HIV. With current US health care financing, multiple measures must be undertaken to fill gaps in coverage. This is especially true for HIV disease, which disproportionately affects poorer populations, causes impoverishment through disability, and is expensive to treat. A Medicaid eligibility expansion for persons with HIV infection could be one key part of increasing access to lifesaving care.
Acknowledgments
This work was supported by the Henry J. Kaiser Family Foundation, with additional support from the National Institute on Drug Abuse via grant DA09531 to the Societal Institute for the Mathematical Sciences.
We thank Elliot Marseille for compilation of HIV progression data; Frederick Hecht for review of our highly active antiretroviral therapy efficacy estimates; Jeffrey Levi, Mike Shriver, Patricia Franks, and Philip Lee for comments on earlier drafts; and 2 anonymous reviewers.
Note. The views expressed are those of the authors and do not necessarily reflect those of the Henry J. Kaiser Family Foundation.
J. G. Kahn designed the analysis, did programming, reviewed data, and wrote the paper. B. Haile did most programming and data review and assisted with analysis design and writing the paper. J. Kates and S. Chang advised on analysis design and portrayal of programs and policies and assisted with writing the paper.
Peer Reviewed
References
- 1.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 2.Lalezari J, Haubrich R, Burger HU, et al. Improved survival and decreased progression of HIV in patients treated with saquinavir (Invirase, SQV) plus HIVID (salcitabine, ddC). In: Program and abstracts of the XI International Conference on AIDS; July 7–12, 1996; Vancouver, British Columbia. Abstract LB.B.6033.
- 3.Murphy R, El-Sadr W, Cheung T, et al. Impact of protease inhibitor containing regimens on the risk of developing opportunistic infections and mortality in the CPCRA 034/ACTG 277 Study. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 181/Session 26.
- 4.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Report of the NIH panel to define principles of therapy of HIV infection and guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. MMWR Morb Mortal Wkly Rep. 1998;47(RR-5):1–41. [PubMed] [Google Scholar]
- 6.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Washington, DC: US Dept of Health and Human Services; 1998.
- 7.Karon JM, Rosenberg PS. Prevalence of HIV infection in the United States, 1984 to 1992. JAMA. 1996;276:126–131. [PubMed] [Google Scholar]
- 8.Gruta CL, Liljestrant P, Balano KB, Legg JJ, Dong BJ, Goldschmidt RH. Antiretroviral usage patterns before and after instituting national treatment guidelines. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 146.
- 9.Colfax G, Hecht F, Chesney MA. Insurance and race predict access to HIV protease inhibitors. In: Program and abstracts of the Sixth Conference on Retroviruses and Opportunistic Infections; February 2–6, 1999; Chicago, Ill. Abstract 106.
- 10.Stolberg SG. AIDS drugs give little hope to thousands unable to pay. New York Times. October 14, 1997:A1.
- 11.Pear R. Expense means many can't get drugs for AIDS. New York Times. February 16, 1997:A1.
- 12.Phillips AN, Smith GD. Viral load and combination therapy for HIV [letter]. N Engl J Med. 1997;336:959. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima AK, Jones JL, Burgess DA, Ward JW. Predictors for not currently receiving protease inhibitor therapy: results from a multisite interview project. In: Program and abstracts of the 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 42282.
- 14.Cunningham WE, Andersen RM, Katz MH. The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37:1270–1281. [DOI] [PubMed] [Google Scholar]
- 15.Bozzette S, Berry S, Duan N, et al. The care of HIV-infected adults in the United States. N Engl J Med. 1998;339:1897–1904. [DOI] [PubMed] [Google Scholar]
- 16.Green J, Arno PS. The “Medicaidization” of AIDS: trends in the financing of HIV-related medical care. JAMA. 1990;264:1261–1266. [DOI] [PubMed] [Google Scholar]
- 17.NASTAD, AIDS Treatment Data Network. National ADAP Monitoring Project: Interim Technical Report. Washington, DC: Henry J. Kaiser Family Foundation; March 1998.
- 18.Steinbrook R. Caring for people with human immunodeficiency virus infection [editorial]. N Engl J Med. 1998;339:1926–1928. [DOI] [PubMed] [Google Scholar]
- 19.Stolberg SG. White House drops plan to cover cost of AIDS drugs for poor. New York Times. December 6, 1997:A10.
- 20.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 21.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86:642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg PS. Scope of the AIDS epidemic in the United States. Science. 1995;270:1372–1375. [DOI] [PubMed] [Google Scholar]
- 23.HIV/AIDS Surveillance Report. Atlanta, Ga: Centers for Disease Control and Prevention; 1997.
- 24.Sweeney PA, Fleming PL, Karon JM, Ward JW. A minimum estimate of the number of living HIV infected persons confidentially tested in the United States [abstract]. In: Poster session: Clinical HIV and HIV Epidemiology: HIV and Other Retroviruses and Complications of AIDS. Presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28–October 1, 1997; Toronto, Ontario.
- 25.Moorman A, Kelaney K, Palella F, Ashman D, HIV Outpatient Study Group. Recent patterns of antiretroviral therapy and clinical response in ambulatory HIV patients. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 144/Session 24.
- 26.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic marker of HIV-1 infection. Ann Intern Med. 1997;126:946–954. [DOI] [PubMed] [Google Scholar]
- 27.Dorrucci M, Pezzotti P, Phillips AN, Alliegro MB, Rezza G. Antiretroviral treatment and progression to AIDS in HIV seroconverters from different risk groups. HIV Italian Seroconversion Study. AIDS. 1997;11:461–467. [DOI] [PubMed] [Google Scholar]
- 28.Eskild A, Magnus P, Sohlberg C, et al. Slow progression to AIDS in intravenous drug users infected with HIV in Norway. J Epidemiol Community Health. 1993;48:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepri AC, Pezzotti P, Dorrucci M, Phillips AN, Rezza G, the Italian Seroconversion Study. HIV disease progression in 854 women and men infected through injecting drug use and heterosexual sex and followed for up to nine years from seroconversion. BMJ. 1994;309:1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaman SR, Brettle RP, Gore SM. Pre-AIDS mortality in the Edinburgh city hospital HIV cohort. Stat Med. 1997;16:2459–2474. [DOI] [PubMed] [Google Scholar]
- 31.Vlahov D, Graham N, Hoover D, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users; plasma viral load and CD4+ cell count. JAMA. 1998;279:35–40. [DOI] [PubMed] [Google Scholar]
- 32.Mariotto AB, Mariotti S, Pezzotti P, Rezza G, Verdecchia A. Estimation of the acquired immunodeficiency syndrome incubation period in intravenous drug users: a comparison with male homosexuals. Am J Epidemiol. 1992;135:428–437. [DOI] [PubMed] [Google Scholar]
- 33.Immunologic markers of AIDS progression: consistency across five HIV-infected cohorts. Multicohort Analysis Project Workshop. Part I. AIDS. 1994;8:911–921. [PubMed] [Google Scholar]
- 34.Pehrson PO, Lindbäck S, Lidman C, Gaines H, Giesecke J. Longer survival after HIV infection for injecting drug users than for homosexual men: implications for immunology. AIDS. 1997;11:1007–1012. [DOI] [PubMed] [Google Scholar]
- 35.Rezza G, Lazzarin A, Angarano G, et al. The natural history of HIV infection in intravenous drug users: risk of disease progression in a cohort of seroconverters. AIDS. 1989;3:87–90. [DOI] [PubMed] [Google Scholar]
- 36.Volberding PA, Lagakos SW, Grimes JM, et al. A comparison of immediate with deferred zidovudine therapy for asymptomatic HIV-infected adults with CD4 cell counts of 500 or more per cubic millimeter. N Engl J Med. 1995;333:401–407. [DOI] [PubMed] [Google Scholar]
- 37.Phillips AN, Elford J, Sabin C, Bofill M, Janossy G, Lee CA. Immunodeficiency and the risk of death in HIV infection. JAMA. 1992;268:2662–2666. [PubMed] [Google Scholar]
- 38.Laraque F, Greene A, Triano-Davis JW, Altman R, Lin-Greenberg A. Effect of comprehensive intervention program on survival of patients with human immunodeficiency virus infection. Arch Intern Med. 1996;156:169–176. [PubMed] [Google Scholar]
- 39.O'Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD, for the VA Cooperative Study Group on AIDS. Changes in plasma HIV RNA levels and CD4+ lymphocyte counts predict both response to antiretroviral therapy and therapeutic failure. Ann Intern Med. 1997;126:939–945. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saravolatz LD, Winslow DL, Collins G, et al. Zidovudine alone or in combination with didanosine or zalcitabine in HIV-infected patients with the acquired immunodeficiency syndrome or fewer than 200 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1099–1106. [DOI] [PubMed] [Google Scholar]
- 42.Apolonio EG, Hoover DR, He Y, et al. Prognostic factors in human immunodeficiency virus-positive patients with a CD4+ lymphocyte count > 50/μL. J Infect Dis. 1995;171:829–836. [DOI] [PubMed] [Google Scholar]
- 43.Darby SC, Ewart DW, Giangrane PLF, Spooner RJD, Rizza CR, for the UK Haemophilia Centre Directors' Organization. Importance of age at infection with HIV-1 for survival and development of AIDS in UK haemophilia population. Lancet. 1996;347:1573–1579. [DOI] [PubMed] [Google Scholar]
- 44.Lundgren JD, Phillips AN, Pedersen C, et al. Comparison of long-term prognosis of patients with AIDS treated and not treated with zidovudine. JAMA. 1994;271:1088–1092. [PubMed] [Google Scholar]
- 45.Osmond D, Charlebois E, Lang W, Shiboski S, Moss A. Changes in AIDS survival time in two San Francisco cohorts of homosexual men, 1983 to 1993. JAMA. 1994;271:1083–1087. [PubMed] [Google Scholar]
- 46.Zangerle R, Reibnegger G, Klein JP. Survival differences in Austrian patients with the acquired immunodeficiency syndrome. Eur J Epidemiol. 1995;11:519–526. [DOI] [PubMed] [Google Scholar]
- 47.Schechter MT, Le N, Craib KJ, Le TN, O'Shaughnessy MV, Montaner JS. Use of the Markov model to estimate the waiting times in a modified WHO staging system for HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:474–479. [DOI] [PubMed] [Google Scholar]
- 48.Chene G, Easterbrook PJ, Juszczak E, Yu LM, Pocock SJ, Gazzard BG. Long-term survival in patients with advanced immunodeficiency. AIDS. 1997;11:209–216. [DOI] [PubMed] [Google Scholar]
- 49.Delta Coordinating Committee. Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996;348:283–291. [PubMed] [Google Scholar]
- 50.d'Arminio-Monforte A, Mainini F, Formenti T, et al. Survival in a cohort of 1205 AIDS patients from Milan [letter]. AIDS. 1996;10:798–799. [DOI] [PubMed] [Google Scholar]
- 51.Ghirardini A, Puopolo M, Rossetti G, et al. Survival after AIDS among Italian haemophiliacs with HIV infection. AIDS. 1995;9:1351–1356. [DOI] [PubMed] [Google Scholar]
- 52.Kitahata MM, Koepsell TD, Deyo RA, Mazwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med. 1996;334:701–706. [DOI] [PubMed] [Google Scholar]
- 53.Turner BJ, Eppes S, McKee LJ, Cosler L, Markson LE. A population-based comparison of the clinical course of children and adults with AIDS. AIDS. 1995;9:65–72. [DOI] [PubMed] [Google Scholar]
- 54.Alcabes P, Munoz A, Vlahov D, Friedland G. Estimation of time from seroconversion to AIDS in HIVinfected intravenous drug users in the US. In: Program and abstracts of the VIII International Conference on AIDS/3rd STD World Congress; July 19–24, 1992; Amsterdam, the Netherlands. Abstract PoC4474.
- 55.Hoover DR, Saah A, Bacellar H, et al. The progression of untreated HIV-1 infection prior to AIDS. Am J Public Health. 1992;82:1538–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook J, Coplan P, Markson L, et al. Rate of AIDS defining events (ADEs) and resulting cost of care: indinavir (IDV) in combination with zidovudine (ZDV) [or stavudine (d4T)] + lamivudine (3TC) versus ZDV [or d4T] + 3TC. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 199/Session 28.
- 57.Curriers R, Williams PL, Grimes JM, Squires KS, Fischl MA, Hammer SM. Incidence rates and risk factors for opportunistic infections in a phase III trial comparing indinavir + ZDV + 3TC to ZDV + 3TC. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 257/Session 37.
- 58.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. [DOI] [PubMed] [Google Scholar]
- 59.Cameron DW, Heath-Chiozzi M, Kravick S, et al. Prolongation of life and prevention of AIDS complication in advanced HIV immunodeficiency with ritonavir update. In: Program and abstracts of the XI International Conference on AIDS; July 7–12, 1996; Vancouver, British Columbia. Abstract Mo.B.411.
- 60.McNaghten AD, Hanson DL, Jones JL, Ward JW, Dworkin MS. The effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 10/Session 6.
- 61.Moore RD, Keruly JC, Chaisson RE. Decline in CMV and other opportunistic disease with combination antiretroviral therapy. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 184/Session 26.
- 62.Sherer R, Pulvirenti J, Cohen M, et al. Six year improvement in HIV hospital outcomes at Cook County Hospital, Chicago: impact of AIDS care units and HAART. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 206/Session 28.
- 63.Wong T, Reggy A, Chiasson MA, Simonds R, Loo V, Heffess J. Protease inhibitors are associated with declining AIDS deaths in New York City. In: Program and abstracts of the 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 12280.
- 64.Thiessard F, Dequae L, Theibaut R, et al. Influence of antiretroviral combinations on disease progression and survival in HIV infected patients, Aquitaine (France), 1988–97. In: Program and abstracts of the 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 22360.
- 65.Hecht FM, Chesney MA, Busch MP, Rawal B, Staprans SI, Kahn JO. Treatment of primary HIV with AZT, 3TC, and indinavir. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 582/Session 77.
- 66.Baruch A, Mastrodonato-Delora P, Schnipper E, Salgo M. Efficacy and safety of triple combination therapy with Invirase (saquinavir/SQV/HIV protease inhibitor), Epivir (3TC/lamivudine) and Retrovir (ZDV/zidovudine) in HIV-infected patients. In: Program and abstracts of the XI International Conference on AIDS; July 7–12, 1996; Vancouver, British Columbia. Abstract Mo. B172.
- 67.Tural C, Clotet B, Peraire J, et al. Double (3TC+d4T) vs triple (3TC+d4T+saquinavir) therapy in HIV-1 experienced patients (ZDV+ddC/ddl) with a low baseline HIV-1 viral load (median 4,098 copies/ml). In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 367/Session 50.
- 68.Hoen B, Harzic M, Dumon B, et al. Efficacy of zidovudine, lamivudine and ritonavir combination in patients with symptomatic primary HIV-1 infection: the ANRS 053/53B trial. Can eradication be obtained? In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 524/Session 66.
- 69.Garcia F, Romeu J, Grau I, et al. An open randomized study to test the influence of maintaining a pre-established viral load reduction in the progression of chronic HIV-1 infected patients in very early stages. In: Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections; February 1–5, 1998; Chicago, Ill. Abstract 330/Session 47.
- 70.Collier AC, Coombs RW, Schoenfeld DA, et al, for the AIDS Clinical Trials Group. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1017. [DOI] [PubMed] [Google Scholar]
- 71.Marschner IC, Collier AC, Coombs RW, et al. Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis. 1998;177:40–47. [DOI] [PubMed] [Google Scholar]
- 72.Katzenstein D, Hammer SM, Hughes MD, et al. The relations of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1091–1098. [DOI] [PubMed] [Google Scholar]
- 73.O'Brien WA, Hartigan PM, Martin D, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–431. [DOI] [PubMed] [Google Scholar]
- 74.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with HIV and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. [DOI] [PubMed] [Google Scholar]
- 75.Foster S, Gregory A, Niederhausen P, et al. Federal HIV/AIDS Spending: A Budget Chartbook. Menlo Park, Calif: Henry J. Kaiser Family Foundation; May 1998.
- 76.Hellinger FJ. The lifetime cost of treating a person with HIV. JAMA. 1993;270:474–478. [PubMed] [Google Scholar]
- 77.Moore RD, Chaisson RE. Costs to Medicaid of advancing immunosuppression in an urban HIV-infected patient population in Maryland. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:223–231. [DOI] [PubMed] [Google Scholar]
- 78.Rietmeijer CA, Davidson AJ, Foster CT, Cohn DL. Cost of care for patients with human immunodeficiency virus infection; patterns of utilization and charges in a public health care system. Arch Intern Med. 1993;153:219–225. [PubMed] [Google Scholar]
- 79.Hellinger FJ, Fleishman JA, Hsia DC. AIDS treatment costs during the 1st months of life: evidence from the ACSUS. Health Serv Res. 1994;29:569–581. [PMC free article] [PubMed] [Google Scholar]
- 80.Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census [letter]. N Engl J Med. 1997;336:1531–1532. [DOI] [PubMed] [Google Scholar]
- 81.Rahman A, Deyton LR, Goetz MB, Rimland D, Simberkoff MS. Inversion of inpatient/outpatient HIV service utilization: impact of improved therapies, clinician education and case management in the US Department of Veterans Affairs. In: Program and abstracts of the 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 433/42429.
- 82.Rawlings JE, Holmes J, Belton B, Selwyn P, Friedland G. Changes in HIV/AIDS patterns of care and estimated costs at an urban medical center during the era of HAART. In: Program and abstracts of the 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 442/42430.
- 83.The 1998 HHS Poverty Guidelines. Washington, DC: US Dept of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation; 1998.
- 84.Garcia F, Vidal C, Gatell JM, Miro JM, Soriano A, Pumarola T. Viral load in asymptomatic patients with CD4+ lymphocyte counts above 500 × 10(6)/1. AIDS. 1997;11:53–57. [DOI] [PubMed] [Google Scholar]
- 85.Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 86.Selden T, Banthin J, Cohen J. Medicaid's problem children: eligible but not enrolled. Health Aff (Millwood). 1998;17:192–200. [DOI] [PubMed] [Google Scholar]
- 87.California Legislative Analyst's Office. A model for health coverage of low-income families. Available at http://www.lao.ca.gov/1999_reports/0699_Low_income_health_coverage.htm. Accessed June 20, 2000.
- 88.Gallant JE. Strategies for long-term success in the treatment of HIV infection. JAMA. 2000;283:1329–1334. [DOI] [PubMed] [Google Scholar]
- 89.Moore RD. Cost effectiveness of combination HIV therapy: three years later. Pharmacoeconomics. 2000;17:325–330. [DOI] [PubMed] [Google Scholar]
- 90.Hornberger J, Aledort JE, Roth N, Eging M, Rose GR, Arnold WE. Early treatment with highly active antiretroviral therapy (HAART) is cost-effective compared to delayed treatment. Poster presented at: 12th World AIDS Conference; June 28–July 3, 1998; Geneva, Switzerland. Abstract 44243.
- 91.Moore RD, Bartlett JG. Combination antiretroviral therapy in HIV infection; an economic perspective. Pharmacoeconomics. 1996;10:109–113. [DOI] [PubMed] [Google Scholar]
- 92.Whitcup SM, Fortin E, Nussenblatt RB, Polic M, Muccioli C, Belfort R Jr. Therapeutic effect of combination antiretroviral therapy on cytomegalovirus retinitis [letter]. JAMA. 1997;277:1519–1520. [PubMed] [Google Scholar]
- 93.Drug company cuts cost of AIDS treatment. New York Times. March 6, 1998:A7.