Injection drug users (IDUs) are at very high risk for infection with hepatitis B virus (HBV) through multiperson use of injection equipment and through unprotected sexual contact. Although a safe and efficacious vaccine exists for hepatitis B, there are multiple problems in vaccinating IDUs in the United States, including (1) discrimination against drug users by health care providers, (2) the need to reach IDUs before they are exposed to HBV, (3) paying for the vaccinations, and (4) difficulties in completing the 3-injection vaccination series.
We compared 2 methods for delivering free hepatitis B vaccination to IDUs: (1) referral by research staff to local health care providers and (2) on-site vaccination at a syringe exchange program.
METHODS
Longitudinal Cohort Study
Funding was obtained to provide hepatitis B vaccination to IDUs in a cohort study conducted in Anchorage, Alaska. Subjects in the study were given counseling and testing for HIV, HBV, and hepatitis C virus. Subjects eligible for hepatitis B vaccination were referred for free vaccinations to 1 of 2 local clinics or to their Medicaid provider. The local clinics instituted a policy of taking study participants before other patients to minimize waiting time.
The research study visits were scheduled for every 6 months and were conducted at a field site in the community. Participants were paid $25 for each interview session and $30 for each session in which testing results were provided. Initial referrals led to a very modest 7% of subjects receiving a first vaccination.1 Additional efforts were then made to increase vaccination. Free transportation was provided from the research site to the local clinics. After November 3, 1997, subjects were paid $10 when they provided proof of each individual vaccination. On March 18, 1999, the monetary incentive for the second and third vaccinations was increased to $20. On April 7, 2000, the incentive was increased to $50 for each vaccination.
Syringe Exchange On-Site Services
Pilot research funding was obtained to study the administration of hepatitis B vaccination at the Positive Health Project, a multiservice syringe exchange program in New York City.
From September 1998 through January 1999, 2 research associates were stationed at the syringe exchange program for 12 hours per week to recruit subjects for the hepatitis B vaccination study. Criteria for participation in the study were (a) having been a participant in the Positive Health Project for at least 1 month and (b) having injected drugs for no longer than 10 years. Informed consent was obtained, a short questionnaire was administered, and a blood sample for HBV testing was taken at the first visit. Subjects received $5 at the first study visit. They returned 1 week later to receive their HBV test results and, if they were eligible for hepatitis B vaccination, to receive their first vaccination; they received $5 at this second study visit. Subjects receiving the vaccine were asked to return 1 month later and 4 months later to receive the second and third injections and for the administration of short follow-up questionnaires. They received $10 at each of these follow-up visits.
Over a 6-month period from September 1998 to February 1999, a physician's assistant or a nurse was available to administer hepatitis B vaccine on 2 days per week for a total of 7 hours. From March 1999 to June 1999, vaccine administration was available for 5 hours on 1 day per week.
RESULTS
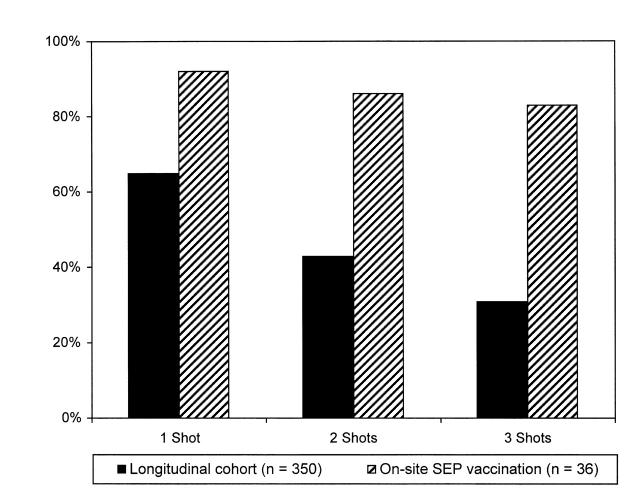
In the cohort study, 350 of 652 subjects had no evidence of previous HBV infection or hepatitis B vaccination and were eligible for vaccination within the study. Figure 1 ▶ shows the final vaccination results after transportation and incentives were implemented—31% of the subjects received 3 shots.
FIGURE 1.
—Hepatitis B vaccination adherence among injection drug users: research longitudinal cohort (n = 350) and syringe exchange program (SEP) site subjects (n = 36).
In the syringe exchange study, 97 persons attending the exchange were asked to participate in the study, of whom 74 (76%) agreed to participate; 36 subjects were HBV negative and in need of vaccination. Figure 1 ▶ also shows the final vaccination outcomes for these 36 subjects—30 of them (83%) received all 3 shots, an adherence comparable to hepatitis B vaccination of IDUs in a study conducted within drug treatment programs2 and to influenza vaccination3 and tuberculosis services4 provided at syringe exchange programs.
These 2 studies suggest that both modest financial incentives and convenient location greatly increase adherence to hepatitis B vaccination among IDUs.
We believe that researchers working with marginalized populations have an ethical obligation to identify better methods for providing health care to those populations, and research funding agencies have an ethical obligation to provide the extra resources required to ensure that research subjects receive the needed health care services.
D. C. Des Jarlais, D. G. Fisher, and J. C. Newman conceived and developed the idea for the brief; developed and refined its content; wrote the first draft and subsequent drafts; discussed the ideas at scientific meetings worldwide; and contributed historical, ethical, and editorial expertise. J. C. Newman and M. Yancovitz in New York and B. N. Trubatch in Alaska coordinated the project, contributed to early and final drafts, provided historical expertise, and coordinated various aspects of supervision of data collection. D. Paone and D. Perlman conceived and developed the idea for the report, developed and refined the intellectual content, wrote and edited numerous drafts, and contributed historical and ethical expertise.
Peer Reviewed
References
- 1.Trubatch BN, Fisher DG, Cagle HH, Fenaughty AM. Vaccination strategies for targeted and difficult-to-access groups. Am J Public Health. 2000;90:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mezzelani P, Venturini L, Turrina G, Lugoboni F, Des Jarlais DC. High compliance with hepatitis B vaccination program among intravenous drug users. J Infect Dis. 1991;163:923–924. [DOI] [PubMed] [Google Scholar]
- 3.Stancliff S, Perlman DC, Salomon N, Russell P. Provision of influenza and pneumococcal vaccines to injection drug users at a syringe exchange. J Subst Abuse Treat. 2000;18:263–265. [DOI] [PubMed] [Google Scholar]
- 4.Perlman DC, Perkins MP, Solomon N, Kochems L, Des Jarlais DC, Paone D. Tuberculosis screening at a syringe exchange program. Am J Public Health. 1997;87:862–863. [PubMed] [Google Scholar]