Abstract
Synaptic activity-dependent de novo gene transcription is crucial for long-lasting neuronal plasticity and long-term memory. In a forebrain neuronal conditional NF-κB-deficient mouse model, we demonstrate here that the transcription factor NF-κB regulates spatial memory formation, synaptic transmission, and plasticity. Gene profiling experiments and analysis of regulatory regions identified the α catalytic subunit of protein kinase A (PKA), an essential memory regulator, as a new NF-κB target gene. Consequently, NF-κB inhibition led to a decrease in forskolin-induced CREB phosphorylation. Collectively, these results disclose a novel hierarchical transcriptional network involving NF-κB, PKA, and CREB that leads to concerted nuclear transduction of synaptic signals in neurons, accounting for the critical function of NF-κB in learning and memory.
New memory formation involves gene expression-dependent changes in synaptic structure and plasticity in the hippocampus (26). Activity-dependent synaptic changes in the brain are thought to be essential components of learning and information storage. Synapses can acquire enhanced or weakened synaptic transmission, depending on the precise synaptic activity pattern (7). Despite the wealth of data showing that neuronal activity influences the morphological organization and function of neuronal networks (35), little is yet known about the molecular signals that translate activity into long-lasting changes.
Nuclear factor-κB (NF-κB) is a ubiquitously expressed transcription factor with posttranslationally regulated activity. Five mammalian NF-κB DNA-binding subunits that can form homo- or heterodimeric combinations have been identified: p50, p52, p65 (Rel-A), c-Rel, and Rel B (18). The role of NF-κB in the immune system and host defense has been well characterized over the last 2 decades (8, 31). In contrast, our understanding of the function of this transcription factor in the central nervous system is just emerging, and its transcriptional neuronal targets have yet to be identified (42, 48, 57). Within the central nervous system, the transcriptionally active form of NF-κB is mostly the p50/p65 heterodimer (23, 51). NF-κB exists in a latent and a “constitutively” active form in neurons (5, 23, 25, 37, 51). The “constitutive” NF-κB identified in several brain regions, such as hippocampus, cortex, and cerebellum, is located within the nucleus and is transcriptionally active. It was reported to be calcium dependent (10, 32, 43) and may be regulated by synaptic activity (2, 25, 41, 43, 51). The latent form is sequestered in the cytoplasm through its interaction with an inhibitory protein IκB. Activation of NF-κB is triggered by multiple stimuli (including glutamate, kainate, amyloid β peptide, tumor necrosis factor alpha [TNF-α], brain injury, oxidative stress, or depolarization) and involves degradation of IκB by the 26S proteasome, allowing nuclear translocation of NF-κB dimers and transcription of target genes (22, 24, 48). As such, NF-κB acts as a signal transducer transmitting information from the active synapse to the nucleus via retrograde transport (23, 41, 43, 56). This observation together with the presence of p65-containing complexes in synaptic terminals raised the prospect of an involvement of NF-κB in the modulation of synaptic function (36, 43).
However, demonstration of the role of NF-κB in neuronal plasticity awaits analysis of neuron-targeted NF-κB-deficient animals. We recently produced a mouse model, in which NF-κB is selectively inhibited in basal forebrain neurons due to tetracycline-dependent overexpression of a transdominant negative mutant, the super-repressor (IκBα-AA) (17). These tTA/IκBα-AA mice present no obvious morphological abnormalities but are more sensitive to excitotoxic stress, which demonstrated the essential role of neuronal NF-κB in the maintenance of adult brain homeostasis (17). Here, we used these cell-specific NF-κB mutant mice to determine the effect of neuronal ablation of NF-κB activity on cognition and synaptic plasticity and analyze genes and pathways involved in learning and memory. We show that NF-κB controls spatial memory formation and synaptic plasticity by regulating the expression of the α catalytic subunit of protein kinase A (PKA) and, consequently, the CREB pathway. These findings thus identify a novel transcriptional signaling cascade in neurons, in which NF-κB regulates the PKA/CREB pathway to function in learning and memory.
MATERIALS AND METHODS
Mice.
The generation of transgenic mice inhibiting NF-κB activity in forebrain neurons was described previously (17). Mice were genotyped by PCR, and to reduce individual variability, only males with an age difference of less than 2 weeks were used in all experiments. Doxycycline (DOX) was given in food at 6 g/kg (Bioserv, Frenchtown, NJ) 14 days before the beginning of the experiments. For in situ hybridization experiments, DOX was administered in drinking water at a concentration of 2 or 6 mg/ml in 2.5% sucrose. The experimenters of behavioral, electrophysiological, and morphological studies were blind to genotype. Animal care was done in accordance with institutional guidelines. Mice were housed in a specific-pathogen free animal house facility on a 12-h light/12-h dark cycle with ad libitum access to rodent chow.
Immunohistochemistry.
Fifteen-micrometer brain coronal cryosections were fixed for 10 min in 4% paraformaldehyde, permeabilized for 1 h in phosphate-buffered saline (PBS), 0.02% Triton X-100, and 2% gelatin and then incubated for 1.5 h at 25°C with polyclonal anti-green fluorescent protein ([GFP] 1:100; Abcam LTD, Cambridge, United Kingdom) and monoclonal anti-neuronal nuclei (NeuN) (1:2,000; Chemicon) or anti-glial fibrillary acidic protein ([GFAP] 1:1,000; Sigma). Anti-mouse immunoglobulin G (IgG) conjugated with Alexa Fluor 488 and anti-rabbit IgG conjugated with Cy3 (1:1,000; Molecular Probes, Interchim, Montluçon, France) were then added for 1 h at 25°C, and immunoreactivities were visualized under an Axiovert 200 M confocal microscope with META deconvolution head-coupled to LSM510 version 3.2 software (Zeiss) settled at Plateforme d'Imagerie Dynamique, Institut Pasteur, Paris.
Behavioral tests.
Behavioral tests were performed using male littermates of 10 to 12 weeks of age. Hippocampus-dependent learning and memory were measured with the Morris water maze test, and nonspatial memory was measured with an object recognition test. The water maze was a circular pool (110 cm in diameter) filled with opacified water at the temperature of 25 ± 0.5°C, located in a quiet room that contained prominent extramaze external cues. Animals were trained over nine training sessions (one per day), each composed of four trials, to find a hidden 7-cm-diameter platform, always situated at the same place at the center of a quadrant of the pool (target quadrant). To begin each trial, the subject was randomly placed in one of five locations in the pool. The animal was allowed 15 s of rest on the platform after finding it (or after being put on the platform by the experimenter if it failed to find it within 60 s). Movement of mice was monitored using an automated tracking system (Videotrack; Viewpoint, France). After full training and 5 days' rest, mice were subjected to a 60-s probe trial in which the platform was removed, and retention was assessed by measuring the percentages of time and distance in the target quadrant. Visual sharpness and motivation were evaluated by two additional trials in which the mice had to find the platform marked by a black and white ping-pong ball at the top of a pole, put in a different quadrant each time. The object recognition test was conducted on the same animals previously challenged in the water maze task. It consisted of a sample trial, during which two similar objects were presented. After 10 min, this trial was followed by a choice trial, during which one of the objects presented at the sample trial (termed a familiar object) was replaced by a new object. The percentage of exploration of the new object was taken as an index of memory. Statistical analysis was done using the Staview program applying analysis of variance with Dunnett's post hoc tests. To confirm the DOX-dependent forebrain expression of the super-repressor and the inhibition of κB binding activity in the double transgenic mice, brain from all individuals were blindly recovered and tested for GFP expression after the behavioral tests. Nuclear extracts from hippocampi were also prepared and analyzed by electrophoretic mobility shift assays. These experiments confirmed the inhibition of NF-κB activity subsequent to the overexpression of the super-repressor in tTA/IκBα-AA double transgenic mice compared to the other groups of mice.
Electrophysiology.
Animals were decapitated after ether anesthesia; the brain was quickly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF). ACSF was saturated with 95% O2-5% CO2 and contained the following (in mM): NaCl, 124.0; KCl, 3.0; KH2PO4, 1.25; NaHCO3, 26.0; MgSO4, 2.0; CaCl2, 2.5; and glucose, 10.0. Hippocampi were isolated and cut into 400-μm-thick transversal slices (29). All recordings were performed at 32°C. Synaptic responses were evoked in the CA3 region of the hippocampus by stimulating Schaffer collaterals with 0.1-ms pulses through monopolar tungsten electrodes. Field excitatory postsynaptic potentials (fEPSPs) were recorded extracellularly in the stratum radiatum of CA1 region using glass microelectrodes (Clark, Reading, England) filled with 3 M NaCl (10 to 20 MΩ). For baseline recordings, slices were stimulated at 0.1 Hz for 20 min at stimulation intensities of 50 to 100 μA. Long-term potentiation (LTP) was induced by applying theta-burst stimulation (TBS). TBS consists of three bursts (10-s interval), each composed of 10 trains (5 Hz) with four pulses (100 Hz) each. Paired-pulse facilitation (PPF) was tested by applying two pulses separated by an interstimulus interval ranging from 10 to 1,000 ms. Long-term depression (LTD) was induced by a paired-pulse low frequency stimulation (ppLFS), which consisted of 900 paired pulses, an interstimulus interval of 20 ms, with 1 Hz.
Pharmacology.
After a 15-min baseline recording, 10 μM DNQX (6,7-dinitroquinoxaline 2,3-dione; Biotrend, Germany) in low Mg2+ (0.5 mM) ACSF was bath applied. After 15 min, 50 μM AP-5 (dl-2-amino-5-phosphonovalerate; Sigma) together with DNQX was added for 20 min to the same slice in low Mg2+ ACSF; afterwards, normal ACSF was used for washout. Statistical analysis was done using Microsoft Excel with a post hoc analysis (Student's t test).
Microarrays.
Design and analysis of customized microarrays were done as previously described (52) with RNA from hippocampus or cortex purified using the QIAGEN RNeasy system (QIAGEN, Hilden, Germany). Samples from multiple animals (n = 4) for each condition (double transgenic mice with or without DOX and single transgenic mice) were analyzed. Printing and postprocessing were performed as previously reported (http://www.microarrays.org/). Data from scanned arrays were obtained using the GenePix software package (Axon Instruments, Union City, CA). The ratios of medians generated for each gene (spot) were intensity dependent normalized according to the following criteria to filter the data: spot size of ≥60 μm, Flag (as in GenePix) value of 0, and signal-to-noise ratio of ≥2.5 in at least one channel. As null hypothesis, we assumed that all genes are not differentially expressed. Thus, a normal distribution of log ratios is expected. After calculation of the mean and standard deviation of ratios using Microsoft Excel, a P value was computed for each ratio.
In situ hybridization.
Digoxigenin (DIG)-labeled riboprobes complementary to the mouse PKA catalytic α gene, sense and antisense, were synthesized using a Roche DIG RNA labeling kit according to the manufacturer's instructions. Cryosections of mouse brain (10 μm) on poly(l-lysine)-coated slides were fixed in 4% paraformaldehyde for 30 min, washed for 5 min in 1× PBS, dehydrated, rehydrated, and finally washed in 1× PBS for 10 min. A total of 10 ng of heat-denatured riboprobe was hybridized to the sections in 100 μl of buffer (3.5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate],10 μg of human Cot1-DNA [Gibco], 1 μg of pol-A RNA [Roche], 2 μg of yeast tRNA [Roche]) at 64°C for 16 h. After four washes of 5 min each in 2× SSC at 25°C and a final wash for 10 min in 1× PBS, sections were then incubated in blocking solution (0.5%, wt/vol; Roche) for 30 min at 25°C in buffer A (100 mM Tris, pH 7.5, 150 mM NaCl) and rinsed in buffer A. Probe was detected with anti-DIG antibody (1:100; Roche) conjugated with alkaline phosphatase for 1 h, followed by washes in 1× PBS and visualization with nitroblue tetrazolium-5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt. Endogenous phosphatase was blocked with 1 mM levimasole (Roche).
Western and Northern blot analysis.
Neuro2a cells constitutively expressing the polycistronic unit coding for the super-repressor and GFP were used to prepare and analyze whole-cell protein extracts as previously reported (19). Total RNA was extracted and analyzed by Northern blotting as previously described (44) with a 32P-radiolabeled probe corresponding to full-length mouse PKAα cDNA cloned by reverse transcription-PCR with the following primers (forward, 5′-ATGGGCAACGCCGCCGCCGCCAAG-3′; reverse, 5′-TAAAACTCAGTAAACTCCTTG-3′) or with a S26 cDNA fragment as an invariant internal control (55).
Electrophoretic mobility shift assay.
Nuclear extracts were prepared, and bandshift assays were performed as previously reported (51), using the canonical κB site derived from the promoter of the major histocompatibility complex class I H-2 Kb gene (KBF1) or the κB site present in intron 2 of the mouse or human PKA catalytic α gene (5′-AGGGGACTCCCCAG-3′ and 5′-AGGGGATGCCCCAG-3′, respectively; the κB site is underlined) as a probe. Functionality of nuclear extracts was assessed by performing bandshift assays for Sp1 (54). Sera raised against p50 (no. 1263) and p65 (no. 1226) were kind gifts of N. Rice (Frederick, MD).
Cell transfection and plasmids.
Wild-type Neuro2a cells were transiently transfected by FuGEN6 (Roche, France) with pGL2 plasmids (Promega, France) containing the minimal cona promoter alone (11) or put downstream of three copies of the κB site from intron 2 of the mouse PKA catalytic α gene, together with an internal control EF1-β-galactosidase, in the presence or absence of expression vectors for transdominant negative mutants of NF-κB. This EF1-β-galactosidase normalization vector was constructed by inserting the HindIII-XbaI 1.2-kb fragment of the human EF1α promoter, blunt-ended for XbaI (47), into the plasmid pSKTNeo (kind gift of S. Tajbakhsh, Institut Pasteur, Paris, France) cut by HindIII and partially by EcoRV. At 48 h after transfection, murine TNF-α (R&D systems, Abingdon, United Kingdom) was added at 10 ng/ml when indicated (see Fig. 5D). After 6 h, cells were lysed in 25 mM Tris phosphate, pH 7.8, 8 mM MgCl2, 1 mM dithiothreitol, 15% glycerol, and 1% Triton; luciferase and β-galactosidase activities were quantified as previously reported (51) for luciferase and with a luminescent β-galactosidase Clontech kit (Ozyme, Saint-Quentin, France) for the β-galactosidase in a Lumat LB 9501 luminometer (Bertold SA, Thoiry, France). Results were then normalized with the β-galactosidase values and expressed as a multiple of control values corresponding to the untreated cells transfected with the conapGL2 construct. Each transfection was done in duplicate in three independent experiments.
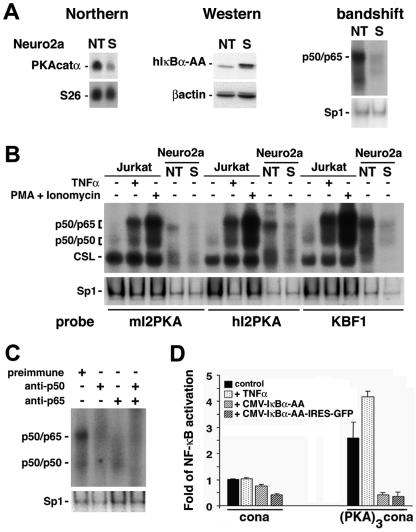
FIG. 5.
PKA catalytic α gene expression is regulated via NF-κB. (A) Northern blot analysis of total RNA (15 μg) from Neuro2a cells nontransfected (NT) or constitutively expressing the super-repressor (S). The top left blot shows hybridization with full-length mouse PKA catalytic α cDNA; in the bottom blot, the same filter was rehybridized with an S26 probe as an invariant control. Western blot (middle) analysis of protein extracts (60 μg) with a polyclonal antibody directed against IκBα (top) or a monoclonal antibody against β-actin as an internal control (bottom) confirms the overexpression of the super-repressor (human IκBα-AA [hIκBα-AA]) in the stable clone (S). Nuclear extracts (5 μg) analyzed by bandshift assay (right) for binding to a canonical κB site show high levels of nuclear p50/p65 heterodimers in nontransfected (NT) Neuro2a cells that are almost totally suppressed in the stable clone (S). The same extracts used for bandshift assays with an Sp1 site serve as an invariant control. (B) Five micrograms of nuclear extracts from Jurkat cells, untreated or treated with TNF-α (10 ng/ml) or with phorbol myristate acetate (PMA; 50 ng/ml) and ionomycin (1 μg/ml), or 0. 5 μg of nuclear extracts from nontransfected Neuro2a cells (NT) or Neuro2a cells stably expressing the super-repressor (S) were analyzed by bandshift assay for their ability to bind to κB sites identified in intron 2 of the mouse and human PKAcatα genes (mI2PKA and hI2PKA, respectively) or to a canonical κB site (KBF1). Variation in specific activities of the probes is responsible for the different intensities observed after binding with the various probes. CSL is CBF1/Su(H)L/Lag1, also known as RBP-Jk complex, which binds a half-κB site and is involved in the Notch pathway. The same extracts used for bandshift assays with an Sp1 site serve as internal controls. (C) Nuclear extracts from Neuro2a cells (5 μg) were preincubated with preimmune serum or sera directed against p50 or/and p65 and analyzed by bandshift assay for binding to the κB site from intron 2 of the mouse PKA catalytic α gene. The same extracts analyzed by bandshift assay for binding to Sp1 serve as internal controls. (D) Neuro2a cells were transfected with luciferase reporter constructs containing a minimal promoter alone (cona) or juxtaposed to a trimerized mouse intron 2 PKA κB site [(PKA)3cona] together with an EF1-lacZ normalization vector, in the absence or presence of expression vectors coding for the super-repressor (CMV-IκBα-AA or CMV-IκBα-AA-IRES-GFP, where IRES is internal ribosome entry site). Cells were left untreated or were treated with TNF-α (10 ng/ml) for 6 h and assessed for activation of the luciferase and β-galactosidase reporter genes. Data are means ± standard errors of the means.
Phosphorylated CREB determination.
Organotypic hippocampal slice cultures were prepared as previously reported (17). After 4 weeks, slices were incubated for 30 min with 10 μM forskolin (Sigma) in fresh ACSF and fixed in 4% paraformaldehyde for two periods of 30 min. Samples were then incubated for 48 h at 4°C with a phospho-CREB (Ser 133)-specific antibody (1:100; Cell Signaling), subsequently detected by indirect immunofluorescence with an anti-rabbit IgG conjugated with Cy3 (1:300; for 48 h at 4°C) (Jackson Laboratory). Images of fluorescence were acquired with a Carl Zeiss Pascal confocal laser scanning microscope. Nuclear phospho-CREB was measured according to Bito et al. (6) and quantified with the National Institutes of Health IMAGEJ program (http://rsb.info.nih.gov/ij/) using the watershed algorithm for segmentation. Statistical analysis of measurements was done with the GraphPad Prism program, applying analysis of variance with Bonferroni's post hoc test.
RESULTS
Neuronal NF-κB loss in tTA/IκBα-AA double transgenic mice.
We previously generated tissue-restricted NF-κB knockout mice through conditional targeting of a transdominant negative mutant of NF-κB, the super-repressor or IκBα-AA, to the forebrain (17). This binary transgenic system in which expression of the super-repressor together with a GFP tracer is tetracycline regulated (Fig. 1A) leads to specific inhibition of both κB binding activity and κB-dependent gene expression (17). Since a precise knowledge of the sites of inhibition of NF-κB activity in the forebrain is a prerequisite to interpret the role of NF-κB in learning and memory, we first analyzed the expression of our inducible transgene, driven by a tetracycline transactivator under the control of a CAMKIIα promoter (39), at the single cell level. We performed immunohistochemical analysis on brain tissue sections from tTA/IκBα-AA double transgenic mice (Fig. 1B). Neuronal cells were identified by their expression of neuronal-specific antigen neuronal nuclei (NeuN), and glial cells were identified by their expression of GFAP (13). Confocal microscopy clearly demonstrated that expression of the inducible transgene in tTA/IκBα-AA double transgenic mice is exclusively neuronal.
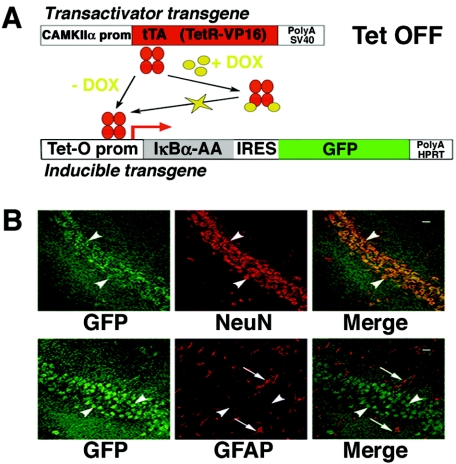
FIG. 1.
Expression of the inducible transgene coding for the super-repressor and GFP in tTA/IκBα-AA double transgenic mice is restricted to neurons. (A) Schematic diagram of the binary inducible transgenic system used to inhibit NF-κB activity in the forebrain. The internal ribosome entry site has been mutated to render GFP a real tracer of super-repressor (IκBα-AA) expression. In the absence of DOX, an analog of tetracycline that passes the blood-brain barrier, super-repressor and GFP are expressed in a polycistronic transcript. SV40, simian virus 40; HPRT, hypoxanthine phosphoribosyltransferase; prom, promoter. (B) Coronal sections from the brain of an 8-week-old tTA/IκBα-AA double transgenic subjected to immunohistochemical analysis with antibodies against GFP and NeuN or GFAP and photographed in the CA1 region. Green, GFP immunoreactivity; red, NeuN immunoreactivity or GFAP immunoreactivity; yellow, double-labeled cells; white arrowheads, neuronal cells; white arrows, glial cells; scale bar, 20 μm.
Neuronal NF-κB loss affects spatial memory formation.
To assess the functional impact of the loss of forebrain neuronal NF-κB in the adult brain, we examined hippocampus-dependent learning and memory tasks in tTA/IκBα-AA double transgenic mice using the Morris water maze. In the four groups (tTA/IκBα-AA double transgenics not treated with doxycycline [DOX] and lacking NF-κB in forebrain neurons; three groups of controls that do not express the super-repressor, i.e., tTA/IκBα-AA double transgenics with DOX and IκBα-AA single transgenics with or without DOX treatment), both the time (Fig. 2A) and the distance (data not shown) to reach the platform significantly decreased across training sessions. After 5 days of rest, when the platform was removed from the maze, percentages of time (Fig. 2B) and distance (data not shown) in the target quadrant were significantly above 25% for all groups, indicating memorization of the location of the platform. tTA/IκBα-AA double transgenics without DOX displayed both a learning impairment, as indicated by a poorer improvement of performance across training sessions (P < 0.05) (Fig. 2A), and a retention impairment, as indicated by lower percentages of time spent (P < 0.002) (Fig. 2B) and distance traveled (P < 0.004) (data not shown) in the target quadrant compared to IκBα-AA single transgenics without DOX. No significant difference between the groups of untreated tTA/IκBα-AA or tTA/IκBα-AA treated with DOX was detected, nor between groups of tTA/IκBα-AA and IκBα-AA with DOX. However, DOX-treated mice regardless of the genotype were indistinguishable (P < 0.87) and the IκBα-AA group with DOX displayed significant differences from IκBα-AA single transgenics (P < 0.036). This indicated that DOX treatment had by itself a significant influence on spatial memory formation that masked the effect specifically due to super-repressor expression. Since we have previously shown that expression of the super-repressor and subsequent inhibition of NF-κB binding activity (17) occur only in tTA/IκBα-AA mice, we consider that the significant difference, observed between tTA/IκBα-AA double transgenics and IκBα-AA single transgenics, is a hallmark of the absence of neuronal NF-κB.
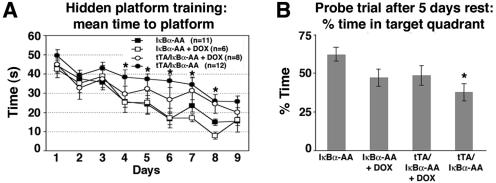
FIG. 2.
Spatial memory formation in the Morris water maze task is impaired in tTA/IκBα-AA double transgenic mice. (A) Every group improved with training; however, significant, longer escape times are observed in tTA/IκBα-AA double transgenic mice in the absence of DOX compared to untreated single transgenic controls (*, P < 0.05). The number of subjects used is indicated in parentheses. DOX treatment of single or double transgenics did not significantly affect learning abilities. (B) Significantly reduced percent time in target quadrant after 5 days' rest in tTA/IκBα-AA double transgenic mice in the absence of DOX relative to untreated single transgenic controls (*, P < 0.002). P values for comparisons for all groups are the following: IκBα-AA versus IκBα-AA+DOX, P < 0.036; IκBα-AA versus tTA/IκBα-AA+DOX, P < 0.066; IκBα-AA+DOX versus tTA/IκBα-AA+DOX, P < 0.87; tTA/IκBα-AA versus IκBα-AA+DOX, P < 0.3; tTA/IκBα-AA versus tTA/IκBα-AA+DOX, P < 0.22; tTA/IκBα-AA versus IκBα-AA, P < 0.002. These values reveal that DOX treatment has by itself a significant influence on memory formation. Data are means ± standard errors of the means.
The memory impairment detected in tTA/IκBα-AA double transgenic mice was not due to poor vision, defective motor abilities, or reduction of motivation since the time to reach the platform was short and comparable for all groups (average, 8 s) when a visible platform was placed in the maze (data not shown). In the object recognition task, all groups of animals spent more time in exploring the new object than the familiar one, indicating that they remembered it. The nonspatial working/episodic memory is therefore unchanged in the double transgenic mutants. Altogether, these results demonstrate that loss of forebrain neuronal NF-κB leads to a significant impairment in spatial memory formation.
Neuronal NF-κB loss impairs synaptic transmission and plasticity.
The cognitive defects exhibited by tTA/IκBα-AA double transgenic mice prompted us to investigate whether synaptic function was affected in these animals. We analyzed the Schaffer CA3-CA1 hippocampal collateral pathway of tTA/IκBα-AA double transgenic mice for deficits in synaptic transmission and two forms of activity-dependent synaptic plasticity, LTP and LTD. To analyze baseline synaptic transmission, we first measured the fEPSP and the amplitude of the fiber volley, which represents a good estimate of the number of stimulated axons in acute hippocampal slices (Fig. 3A). The input-output synaptic relation was significantly reduced at higher stimulus strength (at 0.4 mV fiber volley only) in tTA/IκBα-AA double transgenics compared to IκBα-AA single transgenics (P < 0.05), indicating a slight effect of the absence of neuronal NF-κB on basal synaptic transmission. Again, the DOX treatment worsened performances to an extent that it eliminated the significance of the comparison between the group treated with DOX and the untreated group. PPF, a presynaptic form of short-term plasticity (62), was not significantly different among the groups (Fig. 3B). To specifically analyze the functionality of the N-methyl-D-aspartate (NMDA) receptor, fEPSPs were recorded under low Mg2+ conditions in the presence of the AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptor antagonist DNQX (Fig. 3C). Slices from tTA/IκBα-AA double transgenic mice showed an NMDA receptor component of the fEPSP comparable to control IκBα-AA single transgenics (P > 0.1). This component decreased in all mice tested when the NMDA receptor antagonist AP-5 was added (Fig. 3C). This suggested that NMDA receptor transmission was unaffected in tTA/IκBα-AA double transgenic mice.
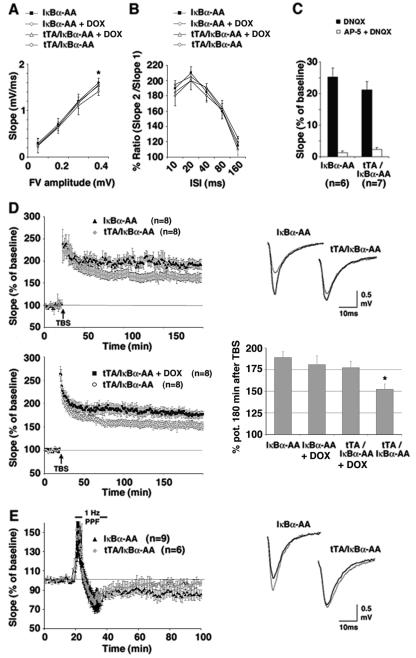
FIG. 3.
Synaptic plasticity in tTA/IκBα-AA double transgenic mice is impaired. (A) The input-output curve was obtained by plotting the fiber volley amplitude against fEPSP slope. At greater fiber volley amplitudes, the slope size in tTA/IκBα-AA double transgenic mice is significantly reduced compared to single transgenic controls (*, P < 0.05; n = 8). (B) PPF in tTA/IκBα-AA double transgenic mice is not significantly affected (P < 0.1; n = 8). (C) Pharmacological isolation of AMPA receptor and NMDA receptor currents by application of DNQX and AP-5, respectively. tTA/IκBα-AA double and IκBα-AA single transgenic mice disclose no significant difference (P > 0.1). (D) Impaired L-LTP in tTA/IκBα-AA double transgenic mice. The top left graph is a summary showing the time course of fEPSPs in tTA/IκBα-AA double transgenics (n = 8) and IκBα-AA single transgenic controls (n = 8). The difference between tTA/IκBα-AA double and IκBα-AA single transgenic mice 100 to 180 min after TBS is significant (P < 0.01). The lower left graph is a summary showing the time course of fEPSPs in tTA/IκBα-AA double transgenics treated with DOX (n = 8) or not treated (n = 8). The difference between untreated and DOX-treated tTA/IκBα-AA double transgenic mice 100 to 180 min after TBS is significant (P < 0.02). Shown at top right are single examples of superimposed traces before TBS application (thin line) and 160 min after the TBS (thick line). The lower right graph is a summary of all LTP experiments with tTA/IκBα-AA double and IκBα-AA single transgenics treated with DOX or not treated (*, P < 0.01 for tTA/IκBα-AA double transgenics versus IκBα-AA single transgenics or P < 0.02 for tTA/IκBα-AA double transgenics versus DOX-treated animals regardless of the genotype). (E) Impaired LTD in tTA/IκBα-AA double transgenic mice. Shown is a summary graph with tTA/IκBα-AA double (n = 6) and IκBα-AA single transgenic mice (n = 9). LTD was induced by ppLFS. The difference between double transgenics and controls 60 min after LFS is significant (P < 0.05). At right are single examples of superimposed traces before ppLFS (thin line) application and 60 min after the ppLFS (thick line). Data are means ± standard errors of the means.
We next explored the effects of neuronal NF-κB loss on synaptic plasticity. Three trains of TBS (46) could induced LTP in tTA/IκBα-AA double transgenic slices. However, its late phase was reduced significantly compared to IκBα-AA single (P < 0.01) or DOX-treated tTA/IκBα-AA double (P < 0.02) transgenic controls 100 to 180 min after TBS application (Fig. 3D). LTD generated by ppLFS, which has been shown to induce LTD efficiently in slices from mature mice (28, 50), was also significantly impaired in tTA/IκBα-AA double transgenics (Fig. 3E). Therefore, forebrain neuronal loss of NF-κB selectively compromises the protein synthesis-dependent late phase of LTP (L-LTP) and prevents induction of LTD. Together, these results show that neuronal NF-κB is involved in activity-dependent synaptic plasticity. NF-κB might regulate genes that are necessary components of the molecular machinery involved in synaptic transmission and plasticity.
Neuronal NF-κB loss selectively down-regulates endogenous gene expression.
To decipher the molecular bases underlying the memory and synaptic function defects of tTA/IκBα-AA double transgenic mice, we next examined the transcriptome of the tTA/IκBα-AA double transgenics overexpressing the super-repressor versus DOX-treated littermates or versus IκBα-AA single transgenics (both similar to wild type) by cDNA microarray analysis. After careful dissection of the hippocampus and cortex, RNA isolation, cDNA synthesis, and Cy3 and Cy5 labeling, probes were hybridized to microarrays as described previously (52). Selected genes with the strongest regulation in cortex and hippocampus are summarized in Table 1. Downregulation of several bona fide NF-κB target genes in the cortex of tTA/IκBα-AA double transgenic mice was found (including junD, HLA class II, or NAIP). Interestingly, regulation of the expression of these target genes appeared to be cortex specific. Strongly enhanced transgenic IκBα expression was specifically detected as an internal control in all microarray experiments in the tTA/IκBα-AA double transgenics compared to DOX-treated double transgenic mice or to single transgenic controls. Standing out from all regulated genes within the hippocampus was the catalytic subunit of cyclic AMP (cAMP)-dependent PKA (PKAcatα) (Table 1, gene symbol PRKACA).
TABLE 1.
Downregulation of selected genes detected by microarray analysis
| Tissue and gene symbol | Accession no. | Relative expression (n-fold) of tTA/IκBα-AA transcriptomea
|
Gene description | |
|---|---|---|---|---|
| With Dox vs without Dox | Vs IκBα-AA | |||
| Cortex | ||||
| HLALS | AF010446 | ↓2.6b | NA | Major histocompatibility complex; class I-like sequence; MR1B |
| BIRC1 | MM004536 | ↓2.6b | NA | Baculoviral IAP repeat-containing 1; neuronal apoptosis inhibitor protein (NAIP) |
| UCHL3 | M30496 | ↓2.5 | ↓1.1 | Ubiquitin carboxyl-terminal esterase L3 (ubiquitin thiolesterase) |
| JUND | X56681 | ↓2.2 | NA | junD proto-oncogene |
| ICAM3 | X69711 | ↓2.1 | ↓1.3 | Intercellular adhesion molecule 3 |
| HLA-DOB | X03066 | ↓2.1 | ↓1.4 | Major histocompatibility complex class II, H2A-D, DO beta |
| NRCAM | 47247 | ↓2.1 | ↓1.6 | Neuronal cell adhesion molecule (NrCAM) |
| NFKBIAd | M69043 | ↑8.6c | ↑7.6c | IκBα |
| Hippocampus | ||||
| PRKACA | X07767 | ↓16.8c | ↓9.4c | Protein kinase, cAMP-dependent, catalytic, alpha |
| NFKBIAd | M69043 | ↑18.1c | ↑11.3c | IκBα |
Arrows indicate up- or downregulation. NA, not applicable.
P < 0.05.
P < 0.001.
Internal positive control.
PKAcatα is a new NF-κB target gene.
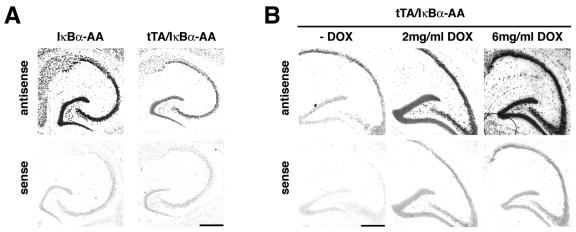
In mice, PKA signaling is critical for learning and memory. L-LTP has been shown to require PKA activity (1) and to be facilitated by constitutively active CREB protein, one of the substrates of PKA (4). PKA downregulation could thus account for the hippocampal-dependent memory and synaptic plasticity defects of our IκBα-AA overexpressing mice. To validate our microarray results, we first performed in situ hybridization studies with a probe specific for PKAcatα. In tTA/IκBα-AA double transgenic mice, the levels of the PKAcatα mRNA were always significantly reduced in comparison to IκBα-AA single transgenic controls (Fig. 4A). Down-regulation of PKAcatα mRNA levels was DOX dose dependent, justifying the use of high doses of DOX to fully inhibit super-repressor expression (Fig. 4B). We next assessed the potential cell autonomous effect of NF-κB inhibition on PKAcatα expression. In a neuronal cell line which constitutively expresses the super-repressor and subsequently presents an inhibition of nuclear NF-κB DNA binding activity, PKAcatα mRNA steady-state levels were strongly decreased (Fig. 5A). We then asked whether this κB-dependent gene regulation was direct or not, and scanned the mouse and human PKAcatα genes for κB sites from 10 kbp upstream to 10 kbp downstream. One κB consensus site (GGGPuNNPyPyCC) which appeared conserved in both species in intron 2 (AGGGGACTCCCCAG in the mouse and AGGGGATGCCCCAG in the human gene; κB sites are underlined) could bind p50/p65 dimers in bandshift assays at the resting state in Neuro2a cells in an NF-κB-dependent manner (compare Neuro2a cells transfected without or with the super-repressor) or after stimulation in Jurkat cells (Fig. 5B). Supershift analysis ascribed to p65/p50 or p50/p50 the complexes bound by the mouse PKA κB site (mI2PKA) in Neuro2a cells (Fig. 5C). When trimerized upstream of a minimal promoter, this mouse PKA κB site specifically drove the expression of a luciferase reporter gene in transient transfection of Neuro2a cells (Fig. 5D). Treatment of cells with a classical NF-κB inducer, such as TNF-α, further enhanced luciferase reporter activity, whereas cotransfection with vectors expressing the super-repressor repressed it completely (Fig. 5C). Taken together, these results demonstrate the κB-dependent regulation of the PKAcatα gene.
FIG. 4.
Expression of PKA catalytic α gene is strongly reduced in tTA/IκBα-AA double transgenic mice. (A) In situ hybridization on brain sagittal sections from a tTA/IκBα-AA double transgenic mouse and an IκBα-AA single transgenic control littermate with an antisense probe specific for the mouse PKA catalytic α gene or with the sense control probe. (B) In situ hybridization with antisense or sense mouse PKA catalytic α gene probes on brain sagittal sections from tTA/IκBα-AA double transgenic mice, untreated or treated with increasing doses of DOX. Scale bar, 200 μm.
Neuronal NF-κB loss reduces forskolin-induced CREB phosphorylation.
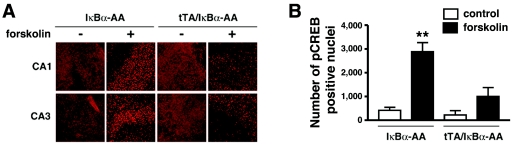
From mollusks to mammals, activation of CREB through Ser133 phosphorylation, in particular by PKA, appears to constitute a crucial step in the molecular switch that converts short- to long-term memory (for reviews, see references 38 and 40). We thus analyzed the level of pCREB in hippocampal slice cultures of tTA/IκBα-AA double transgenic mice or IκBα-AA single transgenic controls in the presence or absence of forskolin, a chemical that activates all adenylyl cyclases and therefore increases cAMP (9). Without treatment, no significant difference was observed between slices regardless of the genotype. Forskolin strongly activated nuclear CREB phosphorylation in the hippocampal fields CA1 and CA3 of control IκBα-AA single transgenic mice. In contrast, forskolin-induced pCREB levels were significantly reduced in tTA/IκBα-AA double transgenics expressing the super-repressor (Fig. 6). These results demonstrate that the PKA/CREB signaling pathway is genuinely affected by the loss of neuronal NF-κB through direct downregulation of the expression of PKAcatα.
FIG. 6.
Inhibition of NF-κB interferes with PKA-dependent CREB phosphorylation. (A) Immunocytochemistry of CREB-phosphorylation on hippocampal slice cultures from tTA/IκBα-AA double transgenic mice or IκBα-AA single transgenic littermate controls, untreated or treated with forskolin for 30 min. CA1, hippocampal region CA1; CA3, hippocampal region CA3. (B) Quantification of the total number of pCREB-positive nuclei in the hippocampal fields observed in panel A (n = 3 mice/genotype). The average values of forskolin-induced CREB phosphorylation are significantly higher in IκBα-AA single transgenic slices relative to tTA/IκBα-AA double trangenics (**, P < 0.01), whereas no significant differences could be observed between forskolin-treated tTA/IκBα-AA double transgenic slices and untreated single (IκBα-AA) or double (tTA/IκBα-AA) transgenics.
DISCUSSION
In this study, we have used transgenic mice to examine the consequences of neuronal abrogation of NF-κB activity on cognition and synaptic plasticity. Combined behavioral, electrophysiological, immunohistochemical, gene profiling, and biochemical analyses demonstrate a direct role of NF-κB in neurons in regulating spatial memory formation, basal synaptic transmission, and activity-dependent synaptic changes. This critical function of NF-κB in cognition and synaptic plasticity is achieved through the control of expression of genes whose products are essential components of the molecular machinery involved in such processes. Indeed, we provide compelling evidence that the PKA catalytic α subunit is a novel NF-κB target gene and that its downregulation in neuron-targeted NF-κB mutant mice affects the CREB pathway.
Previous investigations have suggested a role of NF-κB in synaptic transmission on the basis of its synaptic localization (23, 41), which was recently shown to rely upon the presence of the p65 subunit (43). Studies using κB decoy DNA-treated hippocampal slices or TNF receptor-deficient (TNFR−/−) or p50−/− mice also evoked a link between NF-κB and brain plasticity (2, 27, 36). More recently, LTP was shown to activate NF-κB in mouse hippocampus (16). However, the precise role of NF-κB in neuronal plasticity remained to be demonstrated. We here show that loss of neuronal NF-κB slightly impairs basal synaptic transmission, has a strong effect on the late phase of LTP, and precludes induction of LTD. It is noteworthy that the induction protocol used for the induction of LTD (ppLFS) has been implicated in a protein synthesis-dependent form of LTD, which depends on mGluR (21). Interestingly, LTD induction at 1 Hz is also impaired in TNFR−/− mice, suggesting that the TNFR signaling pathway is important for NF-κB function in synaptic plasticity (2). Meffert et al. reported suppression of NF-κB DNA binding activity by an antagonist of NMDA receptors, AP-5, in hippocampal neuronal cultures (43). This indicated that NF-κB could be activated by basal synaptic transmission through glutamate as proposed earlier in granule cells by Kaltschmidt et al. (24). Using the same antagonist, we now show that NMDA receptor transmission is unchanged in our mouse model, which lacks NF-κB in forebrain neurons. This observation argues against a major role of NF-κB in neurons expressing the subset of glutamate receptors inhibited by this antagonist, which affects preferentially NR2A subunit-containing NMDA receptors (33).
A link between NF-κB and long-term memory was first proposed in crabs (15). It was then reported that p65-deficient mice rescued from embryonic death on a TNFR1−/− background displayed memory defects when challenged in a radial arm maze (43). However, these mice are highly prone to infections and present a deletion of p65 in every cell type, precluding any discrimination between the function of NF-κB in neurons versus glia and complicating the interpretation of behavioral phenotypes. Functional c-Rel binding sites have been lately identified in genes regulated by contextual long-term memory consolidation (30). NF-κB has also been involved in long-term retention of fear memory (58, 59), inhibitory avoidance (14), and retrieval (45). Our present work extends these findings and also demonstrates for the first time that neuronal NF-κB regulates spatial memory formation, whereas nonspatial working/episodic memory is unaltered. This lack of memory impairment in the object recognition task is consistent with recent results indicating that the hippocampus is not involved in short-term object recognition memory (20).
High doses of DOX are required to achieve efficient inhibition of κB-dependent gene expression in vivo (Fig. 4B). Unfortunately, at such high doses, DOX treatment has deleterious effects on either behavioral or electrophysiological experiments, leading to worsened performances in DOX-treated animals, regardless of the genotype, in comparison to single transgenic controls. Our system is conceived such that the absence of DOX induces overexpression of the super-repressor in double transgenic animals. The lack of DOX treatment further decreased behavioral or electrophysiological performances in our double transgenics, thereby stressing the biological impact of the overexpression of the super-repressor. DOX toxicity in controls artificially dampened the extent of the difference of the biological effect due to the super-repressor compared to DOX-treated controls and may lead to nonsignificant values when the biological effect is mild. Therefore, the truly pertinent comparison that should be taken into account here is the comparison between (nontreated) double and single transgenics. We have indeed previously shown that expression of the super-repressor and subsequent inhibition of NF-κB binding activity (17) occurs only in tTA/IκBα-AA mice double transgenics and is not detected in either DOX-treated double transgenic mice or single transgenics.
We showed that loss of neuronal NF-κB drastically down-regulated PKA gene expression in the hippocampus and consequently repressed the PKA-dependent pathway of CREB phosphorylation. These data might help to explain the critical role of NF-κB in behavior and synaptic plasticity, in particular L-LTP, revealed by our mouse model. PKA and CREB have been proposed as critical regulators of long-term memory and L-LTP (4, 12, 38, 49, 53). Recently, the view of CREB as a pivotal component in mouse hippocampal synaptic plasticity and learning has been questioned, since mice with deletion of all CREB isoforms in neurons present normal hippocampal LTP, LTD, and long-term memory (3). These contradictory data could be explained either by a compensation by CREM or by an increase rate of neuronal apoptosis, as described for CREB/CREM mutants (34). However, hypomorphic transdominant negative KCREB mutants, which do not show effects in electrically induced LTP, are deficient in cAMP-mediated long-lasting potentiation (49). It has also been shown that postsynaptic application of a peptide inhibitor of PKA blocks long-lasting potentiation in hippocampal neurons (12); conversely, a constitutively active form of PKAcatα expressed into CA1 pyramidal neurons leads to long-lasting facilitation (4, 12). Altogether, these data indicate that PKAcatα is involved in the long-lasting forms of synaptic plasticity. The downregulation of this protein in our mouse model is probably responsible for the impaired L-LTP. This effect may be mediated through the phosphorylation of CREB and other molecular targets of the PKA. The fact that LTD is absent in tTA/IκBα-AA double transgenic mice suggests that, besides PKA, the expression of other core components of the synaptic machinery might be affected by the loss of neuronal NF-κB. Therefore, the role of NF-κB in synaptic plasticity is likely not due only to PKA and the activation of CREB and involves still unidentified additional signaling pathways as well.
Collectively, our studies demonstrated a role for neuronal NF-κB in cognition and synaptic plasticity. They also disclosed a novel transcriptional cascade where NF-κB controls the CREB signaling pathway via the expression of PKA in neurons. Remarkably, PKA may in turn feed back into NF-κB signaling since IκBα-associated PKAcatα has been shown to phosphorylate p65 through a cAMP-independent mechanism, leading to enhancement of p65 transcriptional activity and interaction with coactivator CREB binding protein/p300 (60, 61). Suppression of PKA-mediated CREB phosphorylation via inhibition of NF-κB might also be relevant for understanding learning impairments in situations where expression of IκBα, an NF-κB target gene, is naturally upregulated, e.g., fever or brain inflammation.
Acknowledgments
We thank C. Cimper and K. Marin for helpful histological assistance, M. Pontoglio for help in genome analysis, S. Tajbakhsh and C. Agulhon for kind gifts of reagents, E. Perret for help with the LSM510 software, and P.-M. Lledo and S. Tajbakhsh for critical reading of the manuscript.
This work is supported in part by grants from the INSERM and Ligue Nationale contre le Cancer (équipe labelisée) to A.I., European Community to A.I. and C.K., Volkswagen-Foundation to C.K. and M.K., Heisenberg-Stipend (DFG) to M.K., Max-Planck-Society to M.K. and V.S., and Institut Pasteur (PTR38) to S.M. S.M. is from the INSERM.
REFERENCES
- 1.Abel, T., P. V. Nguyen, M. Barad, T. A. Deuel, E. R. Kandel, and R. Bourtchouladze. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615-626. [DOI] [PubMed] [Google Scholar]
- 2.Albensi, B. C., and M. P. Mattson. 2000. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35:151-159. [DOI] [PubMed] [Google Scholar]
- 3.Balschun, D., D. P. Wolfer, P. Gass, T. Mantamadiotis, H. Welzl, G. Schutz, J. U. Frey, and H. P. Lipp. 2003. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 23:6304-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barco, A., J. M. Alarcon, and E. R. Kandel. 2002. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108:689-703. [DOI] [PubMed] [Google Scholar]
- 5.Bhakar, A. L., L. L. Tannis, C. Zeindler, M. P. Russo, C. Jobin, D. S. Park, S. MacPherson, and P. A. Barker. 2002. Constitutive nuclear factor-κB activity is required for central neuron survival. J. Neurosci. 22:8466-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bito, H., K. Deisseroth, and R. W. Tsien. 1996. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87:1203-1214. [DOI] [PubMed] [Google Scholar]
- 7.Bliss, T. V. P., and G. L. Collingridge. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31-39. [DOI] [PubMed] [Google Scholar]
- 8.Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-Noriega, L. E., and C. F. Stevens. 1992. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 574:85-92. [DOI] [PubMed] [Google Scholar]
- 10.Cruise, L., L. K. Ho, K. Veitch, G. Fuller, and B. J. Morris. 2000. Kainate receptors activate NF-κB via MAP kinase in striatal neurones. Neuroreport 11:395-398. [DOI] [PubMed] [Google Scholar]
- 11.Dierich, A., M. P. Gaub, J. P. LePennec, D. Astinotti, and P. Chambon. 1987. Cell specificity of the chicken ovalbumin and conalbumin promoters. EMBO J. 6:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy, S. N., and P. V. Nguyen. 2003. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J. Neurosci. 23:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson, P. S., E. Perfilieva, T. Bjork-Eriksson, A. M. Alborn, C. Nordborg, D. A. Peterson, and F. H. Gage. 1998. Neurogenesis in the adult human hippocampus. Nat. Med. 4:1313-1317. [DOI] [PubMed] [Google Scholar]
- 14.Freudenthal, R., M. M. Boccia, G. B. Acosta, M. G. Blake, E. Merlo, C. M. Baratti, and A. Romano. 2005. NF-κB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur. J. Neurosci. 21:2845-2852. [DOI] [PubMed] [Google Scholar]
- 15.Freudenthal, R., and A. Romano. 2000. Participation of Rel/NF-κB transcription factors in long-term memory in the crab Chasmagnathus. Brain Res. 855:274-281. [DOI] [PubMed] [Google Scholar]
- 16.Freudenthal, R., A. Romano, and A. Routtenberg. 2004. Transcription factor NF-κB activation after in vivo perforant path LTP in mouse hippocampus. Hippocampus 14:677-683. [DOI] [PubMed] [Google Scholar]
- 17.Fridmacher, V., B. Kaltschmidt, B. Goudeau, D. Ndiaye, F. M. Rossi, J. Pfeiffer, C. Kaltschmidt, A. Israel, and S. Mémet. 2003. Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J. Neurosci. 23:9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 19.Goudeau, B., F. Huetz, S. Samson, J. P. Di Santo, A. Cumano, A. Beg, A. Israël, and S. Mémet. 2003. IκBα/IκBɛ deficiency reveals that a critical NF-κB dosage is required for lymphocyte survival. Proc. Natl. Acad. Sci. USA 100:15800-15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond, R. S., L. E. Tull, and R. W. Stackman. 2004. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn Mem. 82:26-34. [DOI] [PubMed] [Google Scholar]
- 21.Huber, K. M., M. S. Kayser, and M. F. Bear. 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288:1254-1257. [DOI] [PubMed] [Google Scholar]
- 22.Kaltschmidt, B., M. Uherek, H. Wellmann, B. Volk, and C. Kaltschmidt. 1999. Inhibition of NF-κB potentiates amyloid beta-mediated neuronal apoptosis. Proc. Natl. Acad. Sci. USA 96:9409-9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaltschmidt, C., B. Kaltschmidt, and P. A. Baeuerle. 1993. Brain synapses contain inducible forms of the transcription factor NF-κB. Mech. Dev. 43:135-147. [DOI] [PubMed] [Google Scholar]
- 24.Kaltschmidt, C., B. Kaltschmidt, and P. A. Baeuerle. 1995. Stimulation of ionotropic glutamate receptors activates transcription factor NF-κB in primary neurons. Proc. Natl. Acad. Sci. USA 92:9618-9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltschmidt, C., B. Kaltschmidt, H. Neumann, H. Wekerle, and P. A. Baeuerle. 1994. Constitutive NF-κB activity in neurons. Mol. Cell. Biol. 14:3981-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandel, E. R. 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030-1038. [DOI] [PubMed] [Google Scholar]
- 27.Kassed, C. A., A. E. Willing, S. Garbuzova-Davis, P. R. Sanberg, and K. R. Pennypacker. 2002. Lack of NF-κB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp. Neurol. 176:277-278. [DOI] [PubMed] [Google Scholar]
- 28.Kemp, N., and Z. I. Bashir. 1997. A role for adenosine in the regulation of long-term depression in the adult rat hippocampus in vitro. Neurosci. Lett. 225:189-192. [DOI] [PubMed] [Google Scholar]
- 29.Korte, M., P. Carroll, E. Wolf, G. Brems, H. Thoenen, and T. Bonhoeffer. 1995. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 92:8856-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levenson, J. M., S. Choi, S. Y. Lee, Y. A. Cao, H. J. Ahn, K. C. Worley, M. Pizzi, H. C. Liou, and J. D. Sweatt. 2004. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-Rel. J. Neurosci. 24:3933-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 32.Lilienbaum, A., and A. Israël. 2003. From calcium to NF-κB signaling pathways in neurons. Mol. Cell. Biol. 23:2680-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, L., T. P. Wong, M. F. Pozza, K. Lingenhoehl, Y. Wang, M. Sheng, Y. P. Auberson, and Y. T. Wang. 2004. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:973-974. [DOI] [PubMed] [Google Scholar]
- 34.Mantamadiotis, T., T. Lemberger, S. C. Bleckmann, H. Kern, O. Kretz, A. Martin Villalba, F. Tronche, C. Kellendonk, D. Gau, J. Kapfhammer, C. Otto, W. Schmid, and G. Schutz. 2002. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 31:47-54. [DOI] [PubMed] [Google Scholar]
- 35.Martin, S. J., P. D. Grimwood, and R. G. M. Morris. 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23:649-711. [DOI] [PubMed] [Google Scholar]
- 36.Mattson, M. P., and S. Camandola. 2001. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Investig. 107:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson, M. P., Y. Goodman, H. Luo, W. Fu, and K. Furukawa. 1997. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 49:681-697. [DOI] [PubMed] [Google Scholar]
- 38.Matynia, A., S. A. Kushner, and A. J. Silva. 2002. Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu. Rev. Genet. 36:687-720. [DOI] [PubMed] [Google Scholar]
- 39.Mayford, M., M. E. Bach, Y. Y. Huang, L. Wang, R. D. Hawkins, and E. R. Kandel. 1996. Control of memory formation through regulated expression of a CaMKII transgene. Science 274:1678-1683. [DOI] [PubMed] [Google Scholar]
- 40.Mayford, M., and E. R. Kandel. 1999. Genetic approaches to memory storage. Trends Genet. 15:463-470. [DOI] [PubMed] [Google Scholar]
- 41.Meberg, P. J., W. R. Kinney, E. G. Valcourt, and A. Routtenberg. 1996. Gene expression of the transcription factor NF-κB in hippocampus: regulation by synaptic activity. Mol. Brain Res. 38:179-190. [DOI] [PubMed] [Google Scholar]
- 42.Meffert, M. K., and D. Baltimore. 2005. Physiological functions for brain NF-κB. Trends Neurosci. 28:37-43. [DOI] [PubMed] [Google Scholar]
- 43.Meffert, M. K., J. M. Chang, B. J. Wiltgen, M. S. Fanselow, and D. Baltimore. 2003. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 6:1072-1078. [DOI] [PubMed] [Google Scholar]
- 44.Mémet, S., D. Laouini, J.-C. Epinat, S. T. Whiteside, B. Goudeau, D. Philpott, S. Kayal, P. J. Sansonetti, P. Berche, J. Kanellopoulos, and A. Israël. 1999. IκBɛ-deficient mice: reduction of one T cell precursor sub-species, and enhanced Ig isotype switching and cytokine synthesis. J. Immunol. 163:5994-6005. [PubMed] [Google Scholar]
- 45.Merlo, E., R. Freudenthal, H. Maldonado, and A. Romano. 2005. Activation of the transcription factor NF-κB by retrieval is required for long-term memory reconsolidation. Learn Mem. 12:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minichiello, L., A. M. Calella, D. L. Medina, T. Bonhoeffer, R. Klein, and M. Korte. 2002. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36:121-137. [DOI] [PubMed] [Google Scholar]
- 47.Mizushima, S., and S. Nagata. 1990. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 18:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Neill, L. A., and C. Kaltschmidt. 1997. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20:252-258. [DOI] [PubMed] [Google Scholar]
- 49.Pittenger, C., Y. Y. Huang, R. F. Paletzki, R. Bourtchouladze, H. Scanlin, S. Vronskaya, and E. R. Kandel. 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34:447-462. [DOI] [PubMed] [Google Scholar]
- 50.Saura, C. A., S. Y. Choi, V. Beglopoulos, S. Malkani, D. Zhang, B. S. Shankaranarayana Rao, S. Chattarji, R. J. Kelleher III, E. R. Kandel, K. Duff, A. Kirkwood, and J. Shen. 2004. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42:23-36. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt-Ullrich, R., S. Mémet, A. Lilienbaum, J. Feuillard, M. Raphael, and A. Israël. 1996. NF-κB activity in transgenic mice: developmental regulation and tissue specificity. Development 122:2117-2128. [DOI] [PubMed] [Google Scholar]
- 52.Schwamborn, J., A. Lindecke, M. Elvers, V. Horejschi, M. Kerick, M. Rafigh, J. Pfeiffer, M. Prullage, B. Kaltschmidt, and C. Kaltschmidt. 2003. Microarray analysis of tumor necrosis factor alpha induced gene expression in U373 human glioblastoma cells. BMC Genomics 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva, A. J., J. H. Kogan, P. W. Frankland, and S. Kida. 1998. CREB and memory. Annu. Rev. Neurosci. 21:127-148. [DOI] [PubMed] [Google Scholar]
- 54.Thierry, F., G. Spyrou, M. Yaniv, and P. Howley. 1992. Two AP1 sites binding JunB are essential for human papillomavirus type 18 transcription in keratinocytes. J. Virol. 66:3740-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent, S., L. Marty, and P. Fort. 1993. S26 ribosomal protein RNA: an invariant control for gene regulation experiments in eucaryotic cells and tissues. Nucleic Acids Res. 21:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellmann, H., B. Kaltschmidt, and C. Kaltschmidt. 2001. Retrograde transport of transcription factor NF-κB in living neurons. J. Biol. Chem. 276:11821-11829. [DOI] [PubMed] [Google Scholar]
- 57.West, A. E., E. C. Griffith, and M. E. Greenberg. 2002. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3:921-931. [DOI] [PubMed] [Google Scholar]
- 58.Yeh, S. H., C. H. Lin, and P. W. Gean. 2004. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol. Pharmacol. 65:1286-1292. [DOI] [PubMed] [Google Scholar]
- 59.Yeh, S. H., C. H. Lin, C. F. Lee, and P. W. Gean. 2002. A requirement of nuclear factor-κB activation in fear-potentiated startle. J. Biol. Chem. 277:46720-46729. [DOI] [PubMed] [Google Scholar]
- 60.Zhong, H. H., H. Suyang, H. Erdjument Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]
- 61.Zhong, H. H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]
- 62.Zucker, R. S. 1989. Short-term synaptic plasticity. Annu. Rev. Neurosci. 12:13-31. [DOI] [PubMed] [Google Scholar]