Abstract
Oncogenic signaling stimulates the dynamic remodeling of actin microfilaments and substrate adhesions, essential for cell spreading and motility. Transformation is associated with increased expression of β1,6GlcNAc-branched N-glycans, products of Golgi β1,6-acetylglucosaminyltransferase V (Mgat5) and the favored ligand for galectins. Herein we report that fibronectin fibrillogenesis and fibronectin-dependent cell spreading are deficient in Mgat5−/− mammary epithelial tumor cells and inhibited in Mgat5+/+ cells by blocking Golgi N-glycan processing with swainsonine or by competitive inhibition of galectin binding. At an optimum dosage, exogenous galectin-3 added to Mgat5+/+ cells activates focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3K), recruits conformationally active α5β1-integrin to fibrillar adhesions, and increases F-actin turnover. RGD peptide inhibits PI3K-dependent fibronectin matrix remodeling and fibronectin-dependent cell motility, while galectin-3 stimulates and overrides the inhibitory effects of RGD. Antibodies to the galectin-3 N-terminal oligomerization domain stimulate α5β1 activation and recruitment to fibrillar adhesions in Mgat5+/+ cells, an effect that is blocked by disrupting galectin-glycan binding. Our results demonstrate that fibronectin polymerization and tumor cell motility are regulated by galectin-3 binding to branched N-glycan ligands that stimulate focal adhesion remodeling, FAK and PI3K activation, local F-actin instability, and α5β1 translocation to fibrillar adhesions.
Extracellular matrices (ECM) serve as the molecular scaffold for cell adhesion, migration, proliferation, and differentiation and as a repository of cytokines and molecular cues that determine cell polarization and tissue organization. The deposition of fibronectin (FN) in ECM provides positional information for mesodermal cell migration during early embryogenesis and wound healing (4, 49, 50), and its loss is lethal at embryonic stages (15). FN fibril elongation involves centripetal tensin-dependent translocation of α5β1-integrin from focal adhesions along actin stress fibers, forming fibrillar adhesions that promote conformational changes in soluble FN dimers and assembly of a fibrillar network (37, 47, 52). FN fibrillogenesis is dependent on focal adhesion kinase (FAK) and activation of effector proteins Src kinase and phosphatidylinositol 3-kinase (PI3K) (26, 48). FAK-deficient cells fail to translocate integrin-bound FN along actin filaments to form mature fibrillar adhesions (26). Ilic et al. (26) suggested that the FN fibril defect in Fak−/− cells is due to a failure of postadhesive cytoskeletal reorganization and adhesion site remodeling that is required to support normal FN matrix deposition and patterning.
The traction required for cell motility is also achieved by remodeling of focal adhesions. Integrin receptors cluster in the plane of the membrane and activate Src when engaged by ECM, recruiting paxillin, talin, and vinculin to form complexes that anchor microfilaments. Molecular remodeling of these complexes stimulates membrane endocytosis, pseudopodia extension, and microfilament remodeling (14, 24). Focal adhesions also recruit cytokine receptor tyrosine kinases that collaborate with integrins to activate common oncogenic signaling intermediates including Src, protein kinase Cγ, PI3K, Rac/Cdc42 (30, 43), and the adaptor proteins Grb7, Grb2, and p130cas (20, 42). These observations suggest that fibrillogenesis, cell spreading, and motility share a common requirement for dynamic remodeling of the actin cytoskeleton and focal adhesions. The density of cell surface integrin receptors and of substratum ligands plays a critical role in remodeling rates (36). Therefore, molecular mechanisms that regulate integrin interaction with the substratum should be determinants of fibrillogenesis, spreading, and motility.
In this regard, galectins bind N-acetyllactosamine sequences on N-glycans of integrins (32, 34) and have been described as matricellular molecules that regulate integrin-mediated adhesion to the ECM (27). The preferred ligands for galectins are generated in the Golgi by Mgat5 (β1,6 N-acetylglucosaminyltransferase V), which catalyzes the addition of β1,6GlcNAc to complex type N-glycans, leading to elongation with poly N-acetyllactosamine (Gal-β1,4-GlcNAcn) (3, 22, 39, 51). Mgat5 overexpression increased cell motility and tumor formation (8, 18), while an Mgat5-deficient background suppresses mammary tumor growth and metastasis in polyoma middle T transgenic mice (17, 38). Mammary carcinoma cells from Mgat5+/+ polyomavirus middle T antigen (PyMT) transgenic mice display loss of adhesion junctions and an invasive phenotype in vitro and in vivo. In contrast, tumor cells from Mgat5−/− littermates fail to undergo epithelial-to-mesenchymal transition (EMT) and are insensitive to multiple cytokines and limiting for EMT, including epidermal growth factor, fibroblast growth factor, platelet-derived growth factor, insulin growth factor 1, and transforming growth factor β (38). Herein we report that galectin-3 interactions with Mgat5-modified N-glycans at the cell surface of mammary carcinoma cells stimulate α5β1-integrin activation and translocation to fibrillar adhesions, thereby regulating FN fibrillogenesis and FN-dependent tumor cell spreading and motility.
MATERIALS AND METHODS
Antibodies and reagents.
Bovine plasmatic FN, bovine serum albumin solution (BSA, 30%), swainsonine, β-lactose, RGD peptide, LY294002, rabbit anti-laminin, and mouse anti-β-actin antibodies were purchased from Sigma (Oakville, ON, Canada). Sucrose was purchased from EM industries (Germany). Mouse anti-FN antibody was purchased from Transduction Laboratories (Mississauga, ON, Canada), rat anti-mouse β1-integrin (MAB1997) antibody from Chemicon (Temecula, CA), and rabbit anti-FAK-P397, anti-FAK, anti-Akt ser-473, and anti-Akt antibody from Biosource International (Camarillo, CA). The SNAKA51 anti-α5β1-integrin monoclonal antibody (7) was kindly provided by Martin Humphries, and the monoclonal rat anti-galectin-3 antibody (TIB166) was purchased from the American Type Culture Collection. Fluorescein isothiocyanate- and horseradish peroxidase (HRP)-conjugated rat, mouse, and rabbit secondary antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Phalloidin and secondary antibodies conjugated to Alexa 488, 568, or 647 were purchased from Molecular Probes (Eugene, OR). l-Phytohemagglutinin (l-PHA) gel and l-PHA HRP were purchased from EY Laboratories (San Mateo, CA). Protein G-Sepharose was purchased from Invitrogen Canada, Inc. (Burlington, ON, Canada). Human recombinant galectin-3 was produced as previously described (16).
Cell culture.
Mgat5+/+ and Mgat5−/− epithelial mammary tumor cells were isolated and cloned from Mgat5-deficient mice crossed with mice expressing the PyMT oncogene (17). PyMT Mgat5−/− cells were rescued by infection with a retroviral Mgat5 expression vector (17). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with nonessential amino acids, vitamins, glutamine, penicillin-streptomycin (Canadian Life Technologies), and 10% fetal bovine serum (Immunocorp, Laval, QC, Canada) at 37°C in a humidified 5% CO2-95% air incubator. Where indicated, coverslips were coated with FN by incubation with 200 μl of a solution of 10 μg/ml of FN in phosphate-buffered saline (PBS) for 30 min at room temperature and then washed three times with PBS before the cells were plated.
Immunofluorescence labeling.
Cells were plated on glass coverslips, coated with 10 μg/ml of FN where indicated, at 37°C in a humidified 5% CO2-95% air incubator in complete medium for 5 h and then for 2 days in serum-free medium supplemented, as indicated, with 1 μg/ml swainsonine, 20 mM β-lactose, 20 mM sucrose, 100 μg/ml RGD, 50 μM LY294002, and/or the indicated concentrations of recombinant galectin-3 or anti-galectin-3 antibodies. Cells were washed with PBS (pH 7.4) supplemented with 0.1 mM Ca2+ and 1 mM Mg2+ (PBS/CM) and then fixed with 3% paraformaldehyde for 15 min at room temperature. After fixation, cells were rinsed extensively with PBS and permeabilized with 0.1% Triton X-100 in PBS/CM containing 0.5% BSA for 15 min to reduce nonspecific binding. Subsequent washing and incubations with phalloidin and primary and fluorescent secondary antibodies were done in PBS/CM containing 0.2% BSA. After labeling, the coverslips were mounted in Airvol (Air Products, Inc., Allentown, PA) and viewed with the 63× Plan Apochromat objective (numerical aperture [NA], 1.3) of a Leica TCS-SP1 confocal microscope, the 100× Plan Apochromat objective (NA, 1.35) of an Olympus FV1000 confocal microscope or the 40× PlanNeoFluor objective (NA, 0.75) of a Zeiss Axiophot fluorescence microscope equipped with a Retiga I300 monochrome 10-bit charge-coupled device camera.
FN fibrillogenesis of cells plated on an FN substrate was quantified from images of slides labeled in parallel for each experiment and acquired with equivalent acquisition parameters. Threshold levels were established across the series of slides from each experiment that selected for fibrillar FN and excluded substrate FN labeling using Northern Eclipse (Empix Imaging) or ImagePro (Media Cybernetics) image analysis software. Expression of fibrillar adhesions was quantified by counting the number of cells that expressed elongated, centrally located β1-integrin-positive adhesions passing over the nucleus (14). SNAKA51 labeling was quantified from confocal images of cells stained in parallel for each experiment and acquired with equivalent acquisition parameters. Individual cells were circumscribed, and total fluorescence intensity values per cell were determined.
Cell spreading.
Cells were added to 96-well plates at 1,000/well coated with the indicated concentrations of FN in serum-free DMEM. Cells were incubated at 37°C and, at various times thereafter, fixed with 3.7% formaldehyde for 1 h at room temperature, and after 3 washes with PBS, cells were incubated with tetramethyl rhodamine isothiocyanate-phalloidin (1:1,000) and Hoechst (1:2,000) with 0.2% Triton X-100 for 30 min at room temperature. Cells were scanned with a 10× objective and identified by nuclear stain, and the cell area was quantified by phalloidin staining using the Cellomics scan array cell-spreading algorithm. Data are means ± standard errors of the means (SEM) of results for 500 cells/well.
Actin microfilament turnover.
Cells were plated in 96-well plates coated with 10 μg/ml of FN in complete medium for 5 h and then for 2 days in serum-free medium supplemented or not, as indicated, with 2 μg/ml galectin-3, 100 μg/ml RGD, or both 2 μg/ml galectin-3 and 100 μg/ml RGD. Following treatment for 1 to 20 min with 0.5 μM latranculin A, an actin monomer-binding drug that renders the monomers incompetent for filament formation, cells were fixed with 3% paraformaldehyde for 15 min at room temperature and labeled with Alexa 568-conjugated phalloidin (F-actin) and Hoechst stain (nucleus). Actin microfilament density per cell was determined with a Cellomics Kineticscan HCS reader (10× objective; NA, 0.5) using compartment analysis.
Immunoblot and immunoprecipitation.
For immunoblot experiments, cells cultured at 80% confluence and washed three times with ice-cold PBS/CM were scraped, lysed, and sonicated in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 5 mM EDTA, and 1× Complete Mini (protease inhibitor cocktail tablets) (Roche, Laval, QC, Canada). For immunoprecipitation experiments, cells were scraped and lysed in extraction buffer containing 25 mM Tris, pH 7.5, 25 mM glycine, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and protease inhibitor cocktail. Protein content was assayed using the BCA protein assay (Pierce, Rockford, IL). β1-Integrin was immunoprecipitated with rat anti-integrin β1 and protein G-agarose beads, and β1,6GlcNAc-branched N-glycan proteins were precipitated with l-PHA agarose. To measure FAK-P397 and Akt ser-473 expression, cells were cultured at 60 to 70% confluence on petri dishes coated with 10 μg/ml FN. Cell monolayers were washed three times with ice-cold PBS/CM, lysed with 400 μl of hot (95°C) reducing Laemmli buffer, scraped, and sonicated. Equal protein concentrations were loaded on 7.5% SDS-polyacrylamide gel electrophoresis gels and transferred onto Hybond C Extra nitrocellulose membranes (Amersham). The blots were blocked with 5% nonfat dry milk in PBS containing 0.1% Tween 20, with 5% BSA in TBS (Tris-base, NaCl, pH 7.6) for phosphoprotein blots and with 2% BSA in PBS-0.1% Tween 20 buffer for l-PHA HRP labeling. The labeled bands were revealed by chemiluminescence and exposed to preflashed Kodak XRP-1 film.
Wound healing motility assay.
PyMT mammary tumor cells were plated on 35-mm plastic dishes coated with 10 μg/ml of FN for 2 days at 37°C in a humidified 5% CO2-95% air incubator in complete DMEM until 90% confluence. The cells were grown for 1 day in serum-free medium before wounding of the monolayer by scraping from the middle of the plate and incubation of the cells in serum-free medium supplemented with 20 mM β-lactose, 50 μM LY294002, and indicated concentrations of recombinant galectin-3 and RGD. Cells were then fixed after 1 day, and images were collected with a 20× objective. Images were analyzed, and cell motility was quantified with Northern Eclipse image analysis software by measuring the distance from the scrape of the 10 most motile cells for 4 fields of each condition.
RESULTS
Mgat5/galectin-3-dependent α5β1-integrin activation, FN fibrillogenesis, and FN-dependent cell motility.
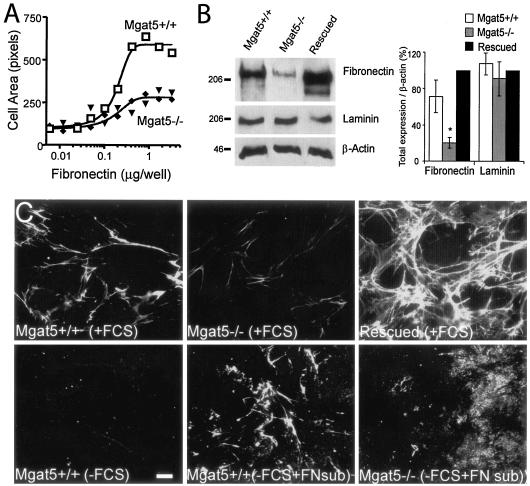
Mgat5+/+ tumor cell spreading increased with FN coating density on the substratum, while Mgat5−/− mammary tumor cells were deficient for spreading on FN (Fig. 1A). This suggested that integrin-dependent functions may be deficient in the Mgat5−/− cells, and to examine this possibility, we measured remodeling of the FN ECM. In Mgat5−/− cells, total cellular levels of FN, but not of laminin, were significantly reduced compared to Mgat5+/+ cells as well as to Mgat5−/− tumor cells rescued by infection with an Mgat5 retroviral vector (referred to as rescued cells) (Fig. 1B). Similarly, expression of FN fibrils was dramatically reduced in Mgat5−/− cells relative to Mgat5+/+ cells (Fig. 1C). As previously observed for sensitivity to cytokines and EMT (38), FN expression is completely restored upon rescue of Mgat5−/− tumor cells by infection with an Mgat5 retroviral vector (Fig. 1B, C). When plated on glass coverslips in serum-depleted medium for 24 h, all cell lines were deficient for FN fibrillogenesis, suggesting that the Mgat5+/+ cells remodel serum FN. Plating the cells in serum-free conditions on an FN substrate was, however, sufficient for Mgat5+/+ but not Mgat5−/− cell lines to reorganize FN into fibrils (Fig. 1C). Mgat5 deficiency, therefore, limits the ability of these mammary tumor cells to remodel exogenous FN.
FIG. 1.
Deficient FN expression and responsiveness in Mgat5−/− cells. (A) Cell spreading of Mgat5+/+ and Mgat5−/− cells plated for 24 h was measured on an FN substrate at the indicated concentrations. (B) Total cell lysates (40 μg protein) of Mgat5+/+ and Mgat5−/− PyMT mammary tumor cell lines and Mgat5−/− cells rescued by retroviral expression of Mgat5 (rescued) were blotted for FN, laminin, and β-actin, as indicated. Molecular mass markers (in kDa) are indicated, and the graph shows the densitometric quantification of band intensity (±SEM; n = 3; *, P < 0.01 relative to Mgat5+/+ cells). (C) Mgat5+/+, Mgat5−/−, and rescued cells grown in serum (plus fetal calf serum [+FCS]) or in the absence of serum (−FCS) on coverslips or on coverslips coated with 10 μg/ml of FN (+FN sub) for 48 h. Cells were fixed and labeled with mouse anti-FN monoclonal antibody and Alexa 568-conjugated anti-mouse secondary antibody. Bar, 20 μm.
Remodeling of substrate FN in serum-free conditions is enhanced in rescued cells exhibiting increased Mgat5 expression relative to Mgat5+/+ cells, and treatments disrupting galectin-glycoprotein cross-linking at the cell surface blocked FN fibrillogenesis (Fig. 2). Treatment of Mgat5+/+ cells with swainsonine, an inhibitor of Golgi α-mannosidase II that prevents processing by Mgat5, or with β-d-lactose, a competitive inhibitor of galectin binding to glycoconjugates, reduces FN fibrillogenesis (Fig. 2). Inhibition of FN fibrillogenesis by β-d-lactose, but not an equivalent concentration of sucrose, and by swainsonine suggests that galectins and their high-affinity ligands (i.e., β1,6GlcNAc-branched N-glycans), respectively, are both required.
FIG. 2.
FN fibrillogenesis is deficient in Mgat5−/− cells. (A) Mgat5+/+, Mgat5−/−, and rescued cells were plated for 2 days on coverslips coated with 10 μg/ml of FN in serum-free medium supplemented with 1 μg/ml swainsonine (SW), 20 mM β-lactose (β-lac), or 20 mM sucrose (Suc), as indicated. Cells were fixed and FN labeled with mouse anti-FN monoclonal antibody, Alexa 488-conjugated antibodies (green), and actin with Texas red phalloidin (red). FN fibril intensity in untreated (white bars) and swainsonine (black bars)-treated cells (B) and from untreated (white bars) and β-lactose (gray bars)- or sucrose (black bars)-treated cells (C) was quantified from 10 images of each condition (±SEM; n = 4; *, P < 0.05; **, P < 0.01 relative to untreated Mgat5+/+ cells [B] and to untreated Mgat5+/+ and rescued cells [C]). Bar, 20 μm.
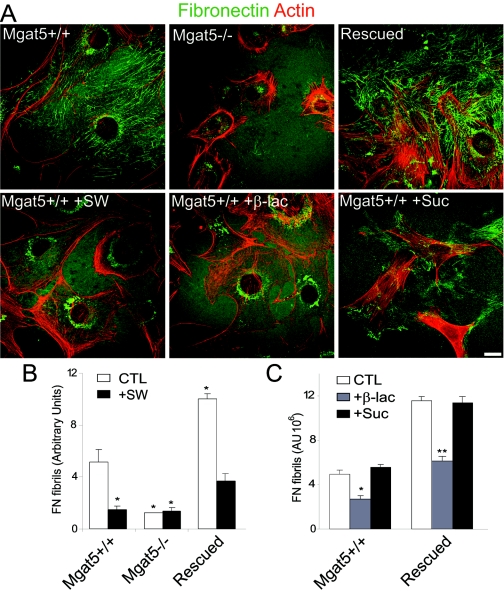
β1-Integrin from Mgat5−/− cells migrated faster in SDS gels than that from Mgat5+/+ and rescued cells, consistent with the presence of β1,6GlcNAc-branched N-glycans on β1-integrin in Mgat5-expressing mammary epithelial tumor cells. Furthermore, β1-integrin in Mgat5-expressing cells but not Mgat5−/− cells reacted with l-PHA, confirming the presence of Mgat5-modified N-glycans (Fig. 3A). Affinity isolation of cellular glycoproteins on l-PHA agarose showed a complete absence of β1-integrin in the bound fraction from Mgat5−/− cells. FN was also detected in the l-PHA isolates from Mgat5+/+ and rescued cells but absent in that of Mgat5−/− cells (Fig. 3B). However, the commercial preparation of soluble FN did not react with l-PHA-HRP, suggesting that Mgat5-modified N-glycans on FN were not required to reorganize exogenous FN into fibrils (Fig. 3B).
FIG. 3.
Mgat5 expression regulates α5β1-integrin glycosylation, conformation, and recruitment to fibrillar adhesions. (A) Total cell lysates, β1-integrin immunoprecipitates, and l-PHA-agarose binding fractions from Mgat5+/+ (WT), Mgat5−/− (−/−), and rescued (Res) cells were blotted with either anti-β1-integrin antibodies, l-PHA-HRP or anti-FN antibodies, as indicated. In Mgat5−/− cells, β1-integrin migrates more rapidly in SDS-polyacrylamide gel electrophoresis (* indicates the integrin band) and is not recognized by the β1,6GlcNAc-branched N-glycan-specific lectin l-PHA. (B) FN also carries l-PHA-labeled β1,6GlcNAc-branched N-glycans (top panel). To determine whether exogenous FN carries β1,6GlcNAc-branched galectin binding sites, 1 μg of commercial FN was blotted with anti-FN antibody or l-PHA-HRP, as indicated (bottom). The multiple FN bands are due to the monomer, dimer, and multimer. Mgat5+/+, Mgat5−/−, and rescued cells were immunofluorescently labeled with anti-β1-integrin (C) or the SNAKA51 monoclonal antibody (D). Cells presenting elongated fibrillar adhesions were counted based on the anti-β1-integrin (C) and total SNAKA51 (D) labeling intensity per cell quantified for each cell type (histogram; ±SEM; n = 3; P values are relative to Mgat5+/+ cells). Bar, 50 μm.
FN fibrillogenesis is associated with the formation of α5β1-integrin-positive fibrillar adhesions that extend along actin stress fibers (52). β1-Integrin-labeled fibrillar adhesions were detected in Mgat5+/+ cells and in rescued cells but not in Mgat5−/− cells (Fig. 3C). Quantification of the number of cells expressing fibrillar adhesions, defined as elongated adhesions that extended over the cell nucleus (14), showed that a minority (∼10%) of wild-type Mgat5+/+ cells expressed fibrillar adhesions, that essentially no fibrillar adhesions were detected in Mgat5−/− cells, but that ∼50% of rescued cells presented fibrillar adhesions (Fig. 3C). We subsequently labeled the cells with the α5 integrin-specific SNAKA51 monoclonal antibody that recognizes an integrin conformation associated with translocation along fibrillar adhesions and FN fibrillogenesis (7). Focal adhesions were labeled with SNAKA51 in both Mgat5+/+ and rescued cells but not in Mgat5−/− cells (Fig. 3D). In rescued cells, SNAKA51 labeling was predominantly associated with elongated fibrillar adhesions. Total SNAKA51 fluorescence labeling in the three cell lines (Fig. 3D) correlated with the expression of fibrillar adhesions (Fig. 3C) and the ability of the cells to remodel substrate FN into fibrils (Fig. 2B).
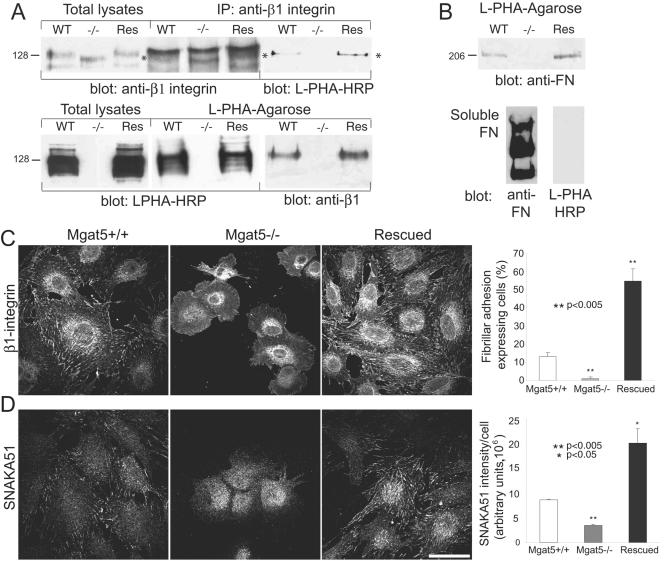
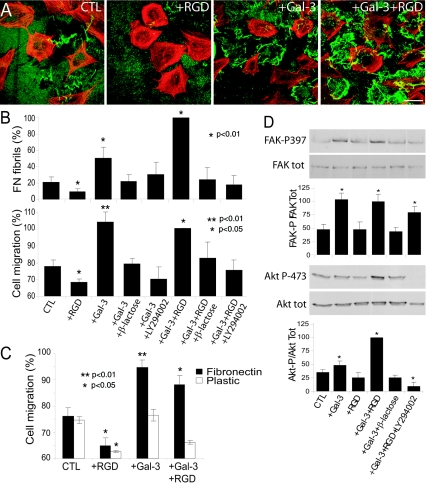
Galectin-3 has a single carbohydrate binding domain and an N terminus interaction domain that mediates multimerization in the presence of high concentrations of multivalent N-glycan ligands (1). The addition of recombinant galectin-3 to the cell medium stimulates FN matrix remodeling and cell motility of Mgat5+/+ cells plated on an FN substrate in a dose-dependent manner with maximum efficiency at a concentration of 1 to 2 μg/ml (Fig. 4). Galectin-3-induced fibrillogenesis was mediated by its carbohydrate binding domain, as it could be inhibited by adding 20 mM β-d-lactose but not 20 mM sucrose (Fig. 4B, inset). At higher concentrations, exogenous galectin-3 stimulated neither FN fibrillogenesis nor cell motility, perhaps because multimerization of the N-terminal domain is inhibited at high concentrations of galectin-3 (Fig. 4). This is analogous to the critical ratio of reactants for optimal cross-linking in multivalent systems, such as antibody immune precipitation. Galectin-3 did not stimulate the motility and FN fibrillogenesis of Mgat5−/− tumor cells (Fig. 4B and C) or the motility of Mgat5+/+ cells plated on tissue culture plastic (Fig. 4D), demonstrating specificity for FN and a requirement for Mgat5-modified N-glycans.
FIG. 4.
Galectin-3 binding to β1,6GlcNAc-branching N-glycans regulates FN fibrillogenesis and cell motility on an FN substrate. (A) Mgat5+/+ cells were plated on an FN substrate in serum-free medium in the absence or presence of 1 μg/ml recombinant galectin-3 and labeled for FN (green) and phalloidin (red). Bar, 20 μm. (B) FN fibril intensity was quantified from 10 images of Mgat5+/+ and Mgat5−/− cells plated in serum-free medium supplemented with 0, 0.5, 1, 2, and 5 μg/ml recombinant galectin-3 (graph; ±SEM; n = 4). FN fibril intensity was also quantified for Mgat5+/+ cells plated in serum-free medium supplemented with 1 μg/ml recombinant galectin-3 (Gal-3), 1 μg/ml Gal-3 plus 20 mM lactose (Gal-3+β-lac), or 20 mM sucrose (Gal-3+Suc) (inset, graph; ±SEM; n = 3). (C) Migration of Mgat5−/− and Mgat5+/+ PyMT tumor cells over a 24-h period in serum-free medium on an FN substrate (10 μg/ml) in the absence or presence of 2 μg/ml galectin-3 was determined using a wound healing assay. The dashed line indicates the location of the wound. Bar, 50 μm. (D) The distance migrated from the wound of the 10 most motile cells was measured in 4 fields for cells plated on plastic or on an FN substrate in the presence of 0, 1, 2, 3, or 5 μg/ml galectin-3 (±SEM; n = 5; *, P < 0.05; **, P < 0.01 relative to Mgat5+/+ cells on FN in the absence of added galectin-3).
RGD peptides enhance the ability of galectin-3 to promote FN fibrillogenesis.
Addition of RGD-containing peptides competes with α5β1-integrin binding to FN (2), and their addition to Mgat5+/+ cells inhibited FN fibrillogenesis and cell motility (Fig. 5A and B). However, in the presence of galectin-3 and RGD peptide, FN fibril formation and cell motility were enhanced, suggesting that exogenous galectin-3 suppresses the inhibitory effects of RGD (Fig. 5A and B). Both cell motility and FN fibrillogenesis induced by galectin-3 or galectin-3 plus RGD were inhibited by treatment with lactose as well as by the PI3K inhibitor LY294002 (Fig. 5B). While the addition of RGD did not significantly affect galectin-3-stimulated motility on an FN substrate, it did inhibit motility in the presence of galectin-3 on plastic (Fig. 5C). The addition of galectin-3 or galectin-3 plus RGD peptides, but not RGD alone, to Mgat5+/+ cells stimulated the phosphorylation of the PI3K substrate Akt and FAK, and galectin-3-dependent activation of both PI3K and FAK was reduced in the presence of lactose (Fig. 5D). While FAK activation by galectin-3 was similar in the presence or absence of RGD peptide, Akt phosphorylation in the presence of galectin-3 was greater with the addition of RGD (Fig. 5D). Galectin-3 therefore stimulates integrin-mediated activation of FAK and PI3K, leading to increased FN fibrillogenesis and cell motility.
FIG. 5.
Galectin-3 stimulates FN fibrillogenesis and cell motility via a PI3K-dependent pathway. (A) To test whether integrin-FN binding regulates galectin-3-induced fibrillogenesis, Mgat5+/+ cells were plated for 2 days on glass coverslips coated with 10 μg/ml FN in serum-free medium (CTL) or in serum-free medium supplemented with 100 μg/ml RGD (+RGD), 1 μg/ml galectin-3 (+Gal-3), or 100 μg/ml RGD and 1 μg/ml galectin-3 (+Gal-3+RGD) and labeled for FN (green) and F-actin (red). Bar, 20 μm. (B) FN fibril intensity (top; 10 images per condition; n = 4; *, P < 0.01 relative to control) and cell migration (bottom; migration of 10 most motile cells from 4 frames for each condition; n = 5; **, P < 0.01; *, P < 0.05 relative to control) were measured in Mgat5+/+ cells plated for 2 days on an FN-coated substrate in serum-free medium supplemented with 100 μg/ml RGD, 1 μg/ml galectin-3, or RGD and galectin-3 in the absence or presence of 20 mM β-lactose or 50 μM LY294002, as indicated. (C) The effect of 100 μg/ml RGD (+RGD), 1 μg/ml galectin-3 (+Gal-3), or 100 μg/ml RGD and 1 μg/ml galectin-3 (+Gal-3+RGD) on cell migration on an FN-coated or plastic substrate was determined (migration of 10 most motile cells from 4 frames for each condition; n = 5; **, P < 0.01; *, P < 0.05 relative to control; P = 0.1079 for Gal-3 versus Gal-3+RGD on FN; P = 0.00621 for Gal-3 versus Gal-3+RGD on plastic). (D) The effect of galectin-3 and/or RGD on FAK and Akt activation was studied by Western blotting of total Mgat5+/+ cell lysates with anti-FAK-P397, anti-FAK, anti-Akt-P473, and anti-Akt antibodies. Phosphorylated and total forms of FAK and Akt were blotted on corresponding antibody-stripped nitrocellulose membranes to determine relative activation in the same samples. The graphs show the densitometric quantification of FAK-P relative to total FAK and Akt-P473 relative to total Akt (n = 3; ±SEM; *, P < 0.01 relative to CTL).
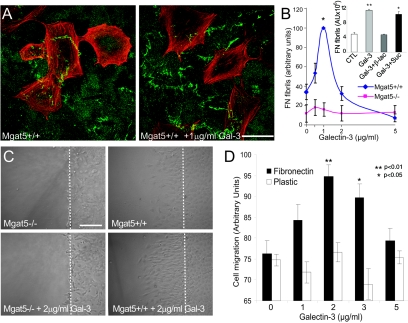
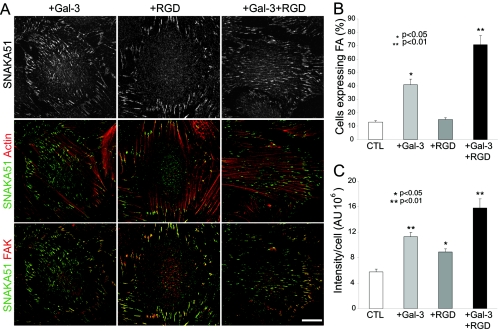
Labeling of Mgat5+/+ cells for β1-integrin and F-actin revealed that RGD treatment alone resulted in the predominant expression of short β1-integrin-positive focal adhesions located at the ends of actin stress fibers. In contrast, addition of galectin-3 or galectin-3 plus RGD induced the formation of elongated fibrillar adhesions that extended along stress fibers over the center of the cell (Fig. 6 A). The number of cells expressing fibrillar adhesions was increased in the presence of galectin-3 and even further in the presence of galectin-3 and RGD (Fig. 6B). The SNAKA51 monoclonal antibody labeled the fibrillar adhesions induced by galectin-3 or by galectin-3 and RGD, and SNAKA51 labeling intensity corresponded to the extent of fibrillar adhesion expression. RGD treatment alone also induced SNAKA51 labeling, which was, however, restricted to FAK-labeled focal adhesions (Fig. 6A and C). Therefore, the activating conformational change in α5β1-integrin detected by SNAKA51 is stimulated, at the concentrations used, by RGD peptides. Following galectin-3 treatment, in the presence or absence of RGD, SNAKA51 labeling extended along fibrillar adhesions from FAK-labeled focal adhesions (Fig. 6A). Therefore, galectin-3 binding enhances conformationally active α5β1-integrin in substrate adhesions and stimulates integrin translocation along fibrillar adhesions.
FIG. 6.
Galectin-3 induces fibrillar adhesions and integrin activation. Mgat5+/+ cells were plated for 2 days on glass coverslips coated with 10 μg/ml of FN in serum-free medium (CTL) or in serum-free medium supplemented with 100 μg/ml RGD (+RGD), 2 μg/ml galectin-3 (+Gal-3), or 100 μg/ml RGD and 2 μg/ml galectin-3 (+Gal-3+RGD). (A) Immunofluorescent labeling of cells for SNAKA51 (top row and in green), F-actin (middle row in red) and FAK (bottom row in red) revealed that RGD-induced SNAKA51 labeling was restricted to FAK-labeled focal adhesions located at the ends of actin stress fibers but that addition of galectin-3, in the presence or absence of RGD, induced the translocation of SNAKA51-labeled α5β1-integrin from FAK-labeled focal adhesions along actin stress fibers. Cells expressing β1-integrin-labeled fibrillar adhesions were counted (B) (n = 3, ±SEM, P values are relative to control) and fluorescent intensity of SNAKA51 antibody labeling determined per cell (C) (n = 3, ±SEM, P values are relative to control). Bar, 20 μm.
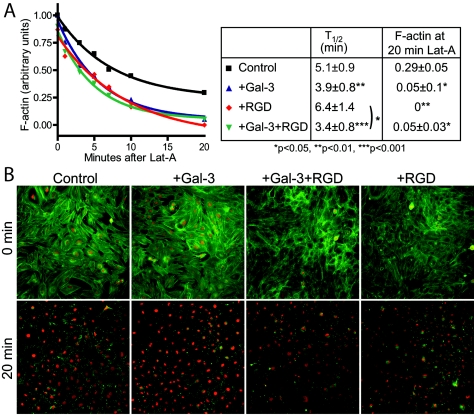
Actin cytoskeleton dynamics are critical for FN fibrillogenesis and translocation of fibrillar adhesions (37, 53). To compare microfilament density and turnover rates, cells attached to fibronectin-coated plates were treated with latrunculin A (Lat-A), an inhibitor of actin polymerization, and loss of microfilament density was measured over time by phalloidin labeling. Mgat5−/− cells display reduced F-actin density and slower decay of actin microfilaments in the presence of Lat-A, consistent with slower remodeling rates in the mutant cells (17). Mgat5+/+ cells grown in the presence of 2 μg/ml galectin-3 for 2 days exhibited a more rapid loss of F-actin upon addition of Lat-A than untreated cells (Fig. 7). In the presence of RGD, microfilament turnover was essentially equivalent to that of untreated cells; however, galectin-3 stimulated actin turnover even in the presence of RGD. Mgat5+/+ cells grown in the presence of RGD peptides presented a reduction in F-actin density after 20 min of Lat-A treatment. The Lat-A stable actin pool was associated with peripheral cell substrate contact regions and was disrupted by treatment with galectin-3 (Fig. 7). Treatment with both RGD and galectin-3 was also associated with reduced actin density, and galectin-3 stimulated actin turnover even in the presence of RGD. Cellular activation by galectin-3 therefore accelerates microfilament dynamics and promotes turnover of a stable RGD-sensitive F-actin pool.
FIG. 7.
Galectin-3 stimulates microfilament dynamics and turnover. (A) Actin filament turnover of Mgat5+/+ cells treated with 2 μg/ml galectin-3 and/or 100 μg/ml RGD peptide (see legend in box at right) was measured by quantifying the density of phalloidin-labeled F-actin per cell following treatment with 0.5 μM Lat-A for 1, 3, 5, 7, 10, and 20 min. F-actin intensities were normalized to maximum and minimum values for each experiment. The half-life of F-actin depolymerization and residual F-actin densities after 20 min of Lat-A treatment are indicated (±SEM; P values are relative to controls unless otherwise indicated; n = 4). (B) Representative images are presented from a Cellomics KSR analysis (10× objective) of phalloidin-labeled Mgat5+/+ cells that were untreated (control) or treated for 2 days with 2 μg/ml galectin-3 (+Gal-3), 100 μg/ml RGD (+RGD), or galectin-3 and RGD (+Gal-3+RGD) prior to (0 min) or 20 min after (20 min) treatment with 0.5 μM Lat-A.
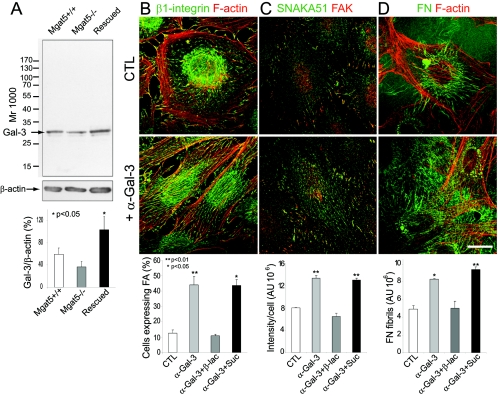
Immunoblotting of cell extracts for galectin-3 revealed that galectin-3 was expressed in Mgat5+/+, Mgat5−/−, and rescued cells with levels slightly reduced in the Mgat5−/− cells but increased in rescued cells relative to Mgat5+/+ cells (Fig. 8A). To demonstrate that endogenous galectin-3 regulates FN fibrillogenesis in Mgat5+/+ cells, we treated the cells with the TIB-166 monoclonal antibody to galectin-3, specific for the N-terminal and not the carbohydrate binding domain of galectin-3 (33). Anti-galectin-3 did not inhibit but rather induced recruitment of β1-integrin to fibrillar adhesions (Fig. 8B) and increased SNAKA51 labeling and localization to fibrillar adhesions (Fig. 8C) as well as FN fibrillogenesis (Fig. 8D). Anti-galectin-3 induced effects could be inhibited by β-d-lactose but not by sucrose, indicating that anti-galectin-3 antibody mediates α5β1-integrin activation and translocation to fibrillar adhesions and FN fibrillogenesis via glycoconjugate binding.
FIG. 8.
Endogenous galectin-3 regulates FN fibrillogenesis and the conformation and association of α5β1-integrin with fibrillar adhesions. (A) Expression of endogenous galectin-3 in Mgat5+/+, Mgat5−/−, and rescued cells was assessed by Western blotting relative to β-actin expression levels. Molecular mass markers (in kDa) are indicated, and the graph shows the densitometric quantification of band intensity (±SEM; n = 4; P values are relative to Mgat5+/+ cells). Mgat5+/+ cells were plated for 2 days on glass coverslips coated with 10 μg/ml of FN in serum-free medium (CTL), in serum-free medium supplemented with anti-galectin-3 monoclonal supernatant (1:50 dilution; +α-Gal-3), or in the presence of anti-galectin-3 antibody plus 20 mM lactose (+α-Gal-3+β-lac) or 20 mM sucrose (+α-Gal-3+Suc). Cells were then labeled for β1-integrin (green) and phalloidin (red) (B), SNAKA51 (green) and FAK (red) (C), or FN (green) and phalloidin (red) (D). Corresponding graphs show the number of cells presenting β1-integrin-labeled fibrillar adhesions (B), SNAKA51 labeling intensity (C), and FN fibril expression (D) in control and treated cells (histogram; ±SEM; n = 3; P values are relative to the control). Addition of control monoclonal antibody to mitochondrial HSP70 had no effect on any of these processes (data not shown). Bar, 10 μm.
DISCUSSION
Galectin-3 binding to Mgat5-modified N-glycans regulates FN-dependent tumor cell motility.
In Mgat5−/− cells, FN-dependent spreading, FN fibrillogenesis, and α5β1-integrin activation and localization to fibrillar adhesions were deficient and rescued by expression of Mgat5. In Mgat5-expressing cells, FN fibrillogenesis could be inhibited by prevention of terminal N-glycosylation with swainsonine or by competitive inhibition of galectin binding with lactose. Galectin-3 was limiting for FN remodeling, α5β1-integrin activation, translocation to fibrillar adhesions, and motility of mammary epithelial tumor cells on an FN substrate but not on plastic. Exogenous galectin-3-induced FAK activation and concomitant addition of RGD peptides enhanced the ability of galectin-3 to induce α5β1-integrin and PI3K activation (7, 26, 40). These results demonstrate that galectin-binding to Mgat5-modified N-glycans induces α5β1-integrin activation, enhancing FN fibrillogenesis and FN-dependent tumor cell spreading and motility. Mgat5-modified N-glycans are present on mature glycoproteins and potentially on many cell surface signaling receptors known to be N-glycosylated.
Galectin-1, -3, and -8 are reported to bind integrins (10, 19, 29), an interaction that might directly promote adhesion remodeling. Galectin-8-coated substratum stimulated integrin-mediated spreading and signaling in a similar fashion to that of cells on FN, while binding of soluble galectin-8 to β1-integrin antagonized adhesion and signaling (27). Increased galectin-3 expression in Mgat5-rescued Mgat5−/− cells is associated with increased α5β1 activation and FN fibrillogenesis (Fig. 2, 3, 8A). Moreover, the ability of anti-galectin-3 antibodies to stimulate these processes in a lactose-dependent manner (Fig. 8) argues that endogenous galectin-3 regulates α5β1 activation and FN fibrillogenesis. Galectin-3 expression was previously reported to be limiting in carcinoma cells, as overexpression enhanced adhesion to laminin, FN, and vitronectin and remodeling of the cytoskeleton and cell spreading, leading to enhanced resistance to apoptotic stimuli (28). However, lactose treatment, a competitive inhibitor of galectin binding, enhanced cell spreading and cytoskeletal reorganization, and this was accompanied by increased endocytosis of galectin-3 and β1-integrins (11), similar in this regard to galectin-3/Mgat5-dependent protection of cytokine receptors from endocytosis (38). The ability of exogenous galectin-3 and anti-galectin-3 antibodies to induce α5β1-integrin conformational changes, F-actin turnover, and FN fibrillogenesis demonstrates that, in these tumor cells, galectin-3 promotes the α5β1-integrin dynamics associated with motility.
In macrophages and transformed cells where membrane remodeling is robust, Mgat5 expression and the galectin lattice are required to maintain sufficient cytokine receptors to drive phagocytosis and the invasive phenotype, respectively (38). The increased loss via constitutive endocytosis and reduced sensitivity of Mgat5−/− tumor cells to stimulation by epidermal growth factor, fibroblast growth factor, platelet-derived growth factor, insulin growth factor 1, and transforming growth factor β argues that galectin-mediated cross-linking regulates the residency and rate of turnover of multiple signaling and adhesion receptors in functional domains. Similarly, at high concentrations, galectin-3 was inhibitory for FN fibrillogenesis, while addition of anti-galectin-3 was stimulatory. This suggests that galectin-3 regulates the dynamics of FN fibrillogenesis and associated integrin activation and translocation via receptor cross-linking. Multivalent galectins bind and cross-link glycoproteins based largely on their N-glycan composition, resulting in heterogeneous clustering of receptors forming a microdomain or lattice with the potential to impact multiple cellular receptors and processes (5). It is therefore possible that galectin secretion regulates FN fibrillogenesis and cell motility through Mgat5 modifications to receptor tyrosine kinases and, in this manner, functions as a regulator of inside-out signaling (24), as described for the ECM protein hensin (23). Alternatively, β1-integrin of Mgat5+/+ cells carries galectin-binding β1,6GlcNAc-branched N-glycans (Fig. 3), and direct binding of galectin-3 could induce α5β1-integrin activation, possibly via integrin clustering within focal contacts.
Stimulation of tumor cell motility by Mgat5/galectin-dependent remodeling of the FN matrix may represent a critical early event in tumor progression. Mgat5 expression plays a critical role at early stages of cell transformation and tumorigenesis (8, 17) and is highly correlated with human colorectal carcinoma progression (9, 44). Cellular transformation is associated with reduction of FN expression in cultured cells (25, 35), although FN expression in tumors varies dramatically (41). Increased expression of β1,6GlcNAc-branched N-glycans upon cellular transformation enables the modulation of integrin-FN interaction by galectin binding. As such, an Mgat5-dependent role for galectin-3 function in cell adhesion and motility would explain the previously reported cell type-specific effects of galectin-3 expression on cell adhesion, cell motility, and tumor metastasis in various cell models (45).
Mgat5/galectin-3-dependent FN fibrillogenesis.
FN fibrillogenesis involves the dissociation of α5β1-integrin from focal adhesions and its translocation along fibrillar adhesions (37). The conformation-dependent SNAKA51 anti-α5 integrin antibody recognizes α5β1-integrin in fibrillar adhesions and also promotes FN fibrillogenesis and induces α5β1-integrin translocation from focal adhesions to fibrillar adhesions (7). Galectin-3 and anti-galectin-3 antibodies each induced increased SNAKA51 binding, confirming that the effect of these treatments on FN fibrillogenesis and cell spreading and motility were coupled to increased α5β1-integrin activation. SNAKA51 binding was also induced by RGD peptide alone but was restricted to focal adhesions and did not stimulate FN remodeling. The addition of RGD peptides disrupts a peripheral Lat-A-resistant F-actin pool detected in untreated Mgat5+/+ mammary epithelial tumor cells (Fig. 7), consistent with ligand-induced integrin regulation of actin polymerization and remodeling in focal complexes (6, 12). Exogenous galectin-3 also diminished the Lat-A-resistant F-actin pool and, in contrast to RGD peptides alone, also stimulated FAK phosphorylation and accelerated the rate of microfilament decay upon addition of Lat-A (Fig. 5, 7). Galectin-3 therefore acts as a dominant enhancer of integrin activation to stimulate both integrin and FAK activation within focal adhesions as well as integrin translocation to fibrillar adhesions and actin filament turnover.
Exogenous RGD peptides have been shown to inhibit cell adhesion to FN, although at lesser concentrations, they can accelerate integrin-mediated remodeling (2, 21). Inhibition of FN fibrillogenesis and cell motility by RGD peptides is abrogated in the presence of galectin-3, where the combination appears to promote integrin activation and turnover. Integrin binding to FN via both RGD-based ligand binding and the synergy domain is associated with FN fibrillogenesis (13, 31, 47). Promotion or stabilization of this alternate non-RGD integrin-FN adhesive mode may be associated with galectin induction of FN fibrillogenesis.
RGD peptides inhibit cell migration and FN fibrillogenesis, while galectin-3 alone stimulates and overrides RGD inhibition of the same. Unlike RGD peptide, galectin-3 stimulated the recruitment of α5β1-integrin to fibrillar adhesions and activation of Akt and FAK phosphorylation. Furthermore, the PI3K inhibitor LY294002 inhibits FN fibrillogenesis but not FAK activation induced by galectin-3, and FAK remains localized to focal adhesions upon galectin-3-induced α5β1-integrin translocation to fibrillar adhesions (Fig. 6). These data suggest that the role of FAK activation in FN fibrillogenesis is at the level of focal adhesion remodeling (26) and represents an early event in the transition of focal adhesions to fibrillar adhesions. Adhesion-dependent phosphorylation of Akt by integrin can occur independently of FAK and Src (46). In the context of galectin-3-induced FN fibrillogenesis, integrin activation of PI3K would appear to be associated with integrin translocation to fibrillar adhesions.
We propose a model whereby galectin-3 cross-linking of glycoprotein receptors promotes α5β1 recruitment and activation within focal adhesions. Galectin-3 is known to bind receptor tyrosine kinases and integrins that contribute via outside-in and inside-out signaling to adhesion remodeling, respectively. Stimulation by galectin-3 results in FAK activation and integrin-substratum exchange that is subsequently coupled to PI3K activation, microfilament turnover, and the recruitment and translocation of integrins to fibrillar adhesions. By promoting both α5β1-integrin activation and actin filament turnover, galectin binding controls the translocation rate of fibrillar adhesion movement along actin stress fibers, FN fibril stretching, and FN polymerization.
Acknowledgments
We are particularly grateful to Martin Humphries for his kind gift of the SNAKA51 monoclonal antibody.
This study was supported by a grant from the Canadian Institutes of Health Research (CIHR) to I.R.N. and J.W.D. and NIH grant CA-46120 to A.R. I.R.N. is an Investigator of the CIHR, and J.W.D. holds a Canada Research Chair. A.L. is the recipient of an FCAR en santé studentship, and J.G.G. holds a doctoral fellowship from the Ministère de la Recherche et des Technologies for his doctoral studies to be submitted jointly to the Université de Montréal and the Université Louis Pasteur de Strasbourg (UMR CNRS 7034).
REFERENCES
- 1.Ahmad, N., H. J. Gabius, S. Andre, H. Kaltner, S. Sabesan, R. Roy, B. Liu, F. Macaluso, and C. F. Brewer. 2004. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279:10841-10847. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu, H., K. Ichihara-Tanaka, K. Ozono, W. Kamiike, H. Matsuda, and K. Sekiguchi. 1996. Suppression of transformed phenotypes of human fibrosarcoma cells by overexpression of recombinant fibronectin. Cancer Res. 56:4541-4546. [PubMed] [Google Scholar]
- 3.Barboni, E. A., S. Bawumia, K. Henrick, and R. C. Hughes. 2000. Molecular modeling and mutagenesis studies of the N-terminal domains of galectin-3: evidence for participation with the C-terminal carbohydrate recognition domain in oligosaccharide binding. Glycobiology 10:1201-1208. [DOI] [PubMed] [Google Scholar]
- 4.Boucaut, J. C., K. E. Johnson, T. Darribere, D. L. Shi, J. F. Riou, H. B. Bache, and M. Delarue. 1990. Fibronectin-rich fibrillar extracellular matrix controls cell migration during amphibian gastrulation. Int. J. Dev. Biol. 34:139-147. [PubMed] [Google Scholar]
- 5.Brewer, C. F., M. C. Miceli, and L. G. Baum. 2002. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12:616-623. [DOI] [PubMed] [Google Scholar]
- 6.Burgstaller, G., and M. Gimona. 2004. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J. Cell Sci. 117:223-231. [DOI] [PubMed] [Google Scholar]
- 7.Clark, K., R. Pankov, M. A. Travis, J. A. Askari, A. P. Mould, S. E. Craig, P. Newham, K. M. Yamada, and M. J. Humphries. 2005. A specific {alpha}5{beta}1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J. Cell Sci. 118:291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetriou, M., I. R. Nabi, M. Coppolino, S. Dedhar, and J. W. Dennis. 1995. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J. Cell Biol. 130:383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes, B., U. Sagman, M. Auger, M. Demetriou, and J. W. Dennis. 1991. β1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res. 51:718-723. [PubMed] [Google Scholar]
- 10.Fischer, C., H. Sanchez-Ruderisch, M. Welzel, B. Wiedenmann, T. Sakai, S. Andre, H. J. Gabius, L. Khachigian, K. M. Detjen, and S. Rosewicz. 2005. Galectin-1 interacts with the {alpha}5{beta}1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 280:37266-37277. [DOI] [PubMed] [Google Scholar]
- 11.Furtak, V., F. Hatcher, and J. Ochieng. 2001. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 289:845-850. [DOI] [PubMed] [Google Scholar]
- 12.Galbraith, C. G., K. M. Yamada, and M. P. Sheetz. 2002. The relationship between force and focal complex development. J. Cell Biol. 159:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, A. J., J. E. Schwarzbauer, and D. Boettiger. 2002. Distinct activation states of alpha5beta1 integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry 41:9063-9069. [DOI] [PubMed] [Google Scholar]
- 14.Geiger, B., A. Bershadsky, R. Pankov, and K. M. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2:793-805. [DOI] [PubMed] [Google Scholar]
- 15.George, E. L., E. N. Georges-Labouesse, R. S. Patel-King, H. Rayburn, and R. O. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119:1079-1091. [DOI] [PubMed] [Google Scholar]
- 16.Gong, H. C., Y. Honjo, P. Nangia-Makker, V. Hogan, N. Mazurak, R. S. Bresalier, and A. Raz. 1999. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 59:6239-6245. [PubMed] [Google Scholar]
- 17.Granovsky, M., J. Fata, J. Pawling, W. J. Muller, R. Khokha, and J. W. Dennis. 2000. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6:306-312. [DOI] [PubMed] [Google Scholar]
- 18.Guo, H. B., Y. Zhang, and H. L. Chen. 2001. Relationship between metastasis-associated phenotypes and N-glycan structure of surface glycoproteins in human hepatocarcinoma cells. J. Cancer Res. Clin. Oncol. 127:231-236. [DOI] [PubMed] [Google Scholar]
- 19.Hadari, Y. R., R. Arbel-Goren, Y. Levy, A. Amsterdam, R. Alon, R. Zakut, and Y. Zick. 2000. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J. Cell Sci. 113(Pt 13):2385-2397. [DOI] [PubMed] [Google Scholar]
- 20.Han, D. C., and J. L. Guan. 1999. Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 274:24425-24430. [DOI] [PubMed] [Google Scholar]
- 21.Harms, B. D., G. M. Bassi, A. R. Horwitz, and D. A. Lauffenburger. 2005. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys. J. 88:1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrick, K., S. Bawumia, E. A. Barboni, B. Mehul, and R. C. Hughes. 1998. Evidence for subsites in the galectins involved in sugar binding at the nonreducing end of the central galactose of oligosaccharide ligands: sequence analysis, homology modeling and mutagenesis studies of hamster galectin-3. Glycobiology 8:45-57. [DOI] [PubMed] [Google Scholar]
- 23.Hikita, C., S. Vijayakumar, J. Takito, H. Erdjument-Bromage, P. Tempst, and Q. Al-Awqati. 2000. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J. Cell Biol. 151:1235-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 25.Hynes, R. O., and J. A. Wyke. 1975. Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology 64:492-504. [DOI] [PubMed] [Google Scholar]
- 26.Ilic, D., B. Kovacic, K. Johkura, D. D. Schlaepfer, N. Tomasevic, Q. Han, J. B. Kim, K. Howerton, C. Baumbusch, N. Ogiwara, D. N. Streblow, J. A. Nelson, P. Dazin, Y. Shino, K. Sasaki, and C. H. Damsky. 2004. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J. Cell Sci. 117:177-187. [DOI] [PubMed] [Google Scholar]
- 27.Levy, Y., R. Arbel-Goren, Y. R. Hadari, S. Eshhar, D. Ronen, E. Elhanany, B. Geiger, and Y. Zick. 2001. Galectin-8 functions as a matricellular modulator of cell adhesion. J. Biol. Chem. 276:31285-31295. [DOI] [PubMed] [Google Scholar]
- 28.Matarrese, P., O. Fusco, N. Tinari, C. Natoli, F. T. Liu, M. L. Semeraro, W. Malorni, and S. Iacobelli. 2000. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 85:545-554. [PubMed] [Google Scholar]
- 29.Moiseeva, E. P., B. Williams, A. H. Goodall, and N. J. Samani. 2003. Galectin-1 interacts with [beta]-1 subunit of integrin. Biochem. Biophys. Res. Commun. 310:1010-1016. [DOI] [PubMed] [Google Scholar]
- 30.Moro, L., L. Dolce, S. Cabodi, E. Bergatto, E. B. Erba, M. Smeriglio, E. Turco, S. F. Retta, M. G. Giuffrida, M. Venturino, J. Godovac-Zimmermann, A. Conti, E. Schaefer, L. Beguinot, C. Tacchetti, P. Gaggini, L. Silengo, G. Tarone, and P. Defilippi. 2002. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277:9405-9414. [DOI] [PubMed] [Google Scholar]
- 31.Mould, A. P., and M. J. Humphries. 2004. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 16:544-551. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa, H., M. Zheng, S. Hakomori, Y. Tsukamoto, Y. Kawamura, and N. Takahashi. 1996. Detailed oligosaccharide structures of human integrin alpha 5 beta 1 analyzed by a three-dimensional mapping technique. Eur. J. Biochem. 237:76-85. [DOI] [PubMed] [Google Scholar]
- 33.Ochieng, J., R. Fridman, P. Nangia-Makker, D. E. Kleiner, L. A. Liotta, W. G. Stetler-Stevenson, and A. Raz. 1994. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 33:14109-14114. [DOI] [PubMed] [Google Scholar]
- 34.Ochieng, J., M. L. Leite-Browning, and P. Warfield. 1998. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 246:788-791. [DOI] [PubMed] [Google Scholar]
- 35.Olden, K., and K. M. Yamada. 1977. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell 11:957-969. [DOI] [PubMed] [Google Scholar]
- 36.Palacek, S. P., J. C. Loftus, M. H. Ginsberg, D. A. Lauffenberger, and A. F. Horwitz. 1997. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385:537-540. [DOI] [PubMed] [Google Scholar]
- 37.Pankov, R., E. Cukierman, B. Z. Katz, K. Matsumoto, D. C. Lin, S. Lin, C. Hahn, and K. M. Yamada. 2000. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148:1075-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Partridge, E. A., C. Le Roy, G. M. Di Guglielmo, J. Pawling, P. Cheung, M. Granovsky, I. R. Nabi, J. L. Wrana, and J. W. Dennis. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306:120-124. [DOI] [PubMed] [Google Scholar]
- 39.Perillo, N. L., M. E. Marcus, and L. G. Baum. 1998. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J. Mol. Med. 76:402-412. [DOI] [PubMed] [Google Scholar]
- 40.Reiske, H. R., S. C. Kao, L. A. Cary, J. L. Guan, J. F. Lai, and H. C. Chen. 1999. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274:12361-12366. [DOI] [PubMed] [Google Scholar]
- 41.Ruoslahti, E. 1984. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 3:43-51. [DOI] [PubMed] [Google Scholar]
- 42.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435-478. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz, M. A., and M. H. Ginsberg. 2002. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4:E65-E68. [DOI] [PubMed] [Google Scholar]
- 44.Seelentag, W. K., W. P. Li, S. F. Schmitz, U. Metzger, P. Aeberhard, P. U. Heitz, and J. Roth. 1998. Prognostic value of beta1,6-branched oligosaccharides in human colorectal carcinoma. Cancer Res. 58:5559-5564. [PubMed] [Google Scholar]
- 45.Takenaka, Y., T. Fukumori, and A. Raz. 2004. Galectin-3 and metastasis. Glycoconj. J. 19:543-549. [DOI] [PubMed] [Google Scholar]
- 46.Velling, T., S. Nilsson, A. Stefansson, and S. Johansson. 2004. beta1-Integrins induce phosphorylation of Akt on serine 473 independently of focal adhesion kinase and Src family kinases. EMBO Rep. 5:901-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wierzbicka-Patynowski, I., and J. E. Schwarzbauer. 2003. The ins and outs of fibronectin matrix assembly. J. Cell Sci. 116:3269-3276. [DOI] [PubMed] [Google Scholar]
- 48.Wierzbicka-Patynowski, I., and J. E. Schwarzbauer. 2002. Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J. Biol. Chem. 277:19703-19708. [DOI] [PubMed] [Google Scholar]
- 49.Winklbauer, R., and M. Nagel. 1991. Directional mesoderm cell migration in the Xenopus gastrula. Dev. Biol. 148:573-589. [DOI] [PubMed] [Google Scholar]
- 50.Yost, H. J. 1992. Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature 357:158-161. [DOI] [PubMed] [Google Scholar]
- 51.Yu, F., R. L. Finley, Jr., A. Raz, and H. R. Kim. 2002. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J. Biol. Chem. 277:15819-15827. [DOI] [PubMed] [Google Scholar]
- 52.Zamir, E., and B. Geiger. 2001. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 114:3583-3590. [DOI] [PubMed] [Google Scholar]
- 53.Zamir, E., M. Katz, Y. Posen, N. Erez, K. M. Yamada, B. Z. Katz, S. Lin, D. C. Lin, A. Bershadsky, Z. Kam, and B. Geiger. 2000. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2:191-196. [DOI] [PubMed] [Google Scholar]