Abstract
Thyroid hormones, T3 and T4, are known regulators of intestine development. The best characterized example is the remodeling of the gastrointestinal tract during amphibian metamorphosis. Thyroid hormones act via nuclear receptors, the TRs, which are T3-dependent transcription factors. We previously showed that intestinal epithelial cell proliferation is controlled by thyroid hormones and the TRα gene. To analyze the mechanisms responsible, we studied the expression of genes belonging to and/or activated by the Wnt/β-catenin pathway, a major actor in the control of physiological and pathological epithelial proliferation in the intestine. We show that T3-TRα1 controls the transcription of the β-catenin gene in an epithelial cell-autonomous way. This is parallel to positive regulation of proliferation-controlling genes such as type D cyclins and c-myc, known targets of the Wnt/β-catenin. In addition, we show that the regulation of the β-catenin gene is direct, as TR binds in vitro and in chromatin in vivo to a specific thyroid hormone-responsive element present in intron 1 of this gene. This is the first report concerning in vivo transcriptional control of the β-catenin gene. As Wnt/β-catenin plays a crucial role in intestinal tumorigenesis, our observations open a new perspective on the study of TRs as potential tumor inducers.
The mammalian gut develops from the association of the endoderm and the splanchnic mesoderm. The adult features are acquired during well-defined fetal and postnatal developmental steps, which are completed at the weaning (16, 18). The pluristratified gut endoderm is remodeled to form a monolayer of rapidly renewing columnar epithelial cells, organized in the small intestine in the typical finger-shaped structure (49). From a functional point of view, this defines specific spatial and functional units: the villi, which constitute the differentiated compartment, and the crypts of Lieberkühn, where proliferation takes place. The main characteristic of the intestinal epithelium is its continuous cell renewal, which is fuelled by multipotent stem cells within the crypts (49). The rate of crypt cell division, migration, and villus cell differentiation must be tightly controlled to ensure epithelial homeostasis (49).
Several pathways have been described to play an important role in the control of epithelial proliferation in the intestine, during early development as well as during the continuous renewing in adulthood (3, 49). Among these pathways, the Wnt/β-catenin pathway has received major attention, as it is involved in the normal and in the pathological proliferation of the gut (4, 50). The signal transducing components of the Wnt pathway are receptors belonging to the frizzled and LRP protein families (35, 42). One target of the Wnt pathway is the β-catenin protein. In the absence of Wnt signal, β-catenin is destabilized by phosphorylation from a cytoplasmic complex comprising axin, adenomatous polyposis coli, and glycogen synthase kinase 3β. The phosphorylated β-catenin is finally degraded by the proteasome. The action of this complex can be antagonized by disheveled, a cytoplasmic protein that is activated by the frizzled receptors. In addition to Wnt, some other factors have been shown to act in the stabilization of β-catenin, such as insulin-like growth factor, epidermal growth factor, and human growth factor, through their respective thyrosin kinase-associated receptors (35). The main characteristic of the stabilized β-catenin is its accumulation in the cytoplasm and migration into the nucleus, where it acts as a transcriptional coactivator by associating with transcription factors of the Tcf/Lef family (35). Intestinal tumors characterized by actively proliferating cells display high levels of nuclear β-catenin (42). Recently, van de Wetering et al. (52) described the presence of β-catenin in the nuclei of crypt cells, strongly supporting its role in the control of cell proliferation. The complex β-catenin/Tcf has been shown to control the expression of several genes involved in cell cycle/proliferation control like cyclin D1, c-myc, and cdx1 (54) as well as, indirectly, cyclin D2 (20). It is worth noting that mutations of the different partners of this pathway are responsible for tumorigenesis in humans, as they constitutively activate uncontrolled cell proliferation (42, 50). In fact, activation of this pathway as well as that of the ras oncogene are sufficient to induce colon tumorigenesis (3). Surprisingly, to date, very little is known on the transcriptional regulation of the β-catenin gene.
Several observations suggested that thyroid hormones (TH), T3 and T4, are involved in the control of intestinal development (15). First of all, it has been shown that the gastrointestinal tract remodeling during amphibian metamorphosis is completely dependent on TH (48). In rodents, they have been shown to participate in the developmental processes responsible for the increase in the mucosa growth as well as in the onset of the adult-type digestive enzymes of the absorptive enterocytes at weaning (15). The action of TH is mediated by T3 binding to thyroid hormone nuclear receptors, the TRs, which belong to the nuclear hormone receptor family of transcription factors (21). The TRs are encoded by two genes, TRα and TRβ (22), each of which produces several protein isoforms (10). More recently, using congenital hypothyroid and TR knockout mice, we pointed out that TH act on the intestinal postnatal development mainly through the control of the proliferation of the intestine epithelial progenitor cells. This function depends on the TRα1 receptor (9, 13, 41).
To analyze the molecular targets of T3-activated TRα1 in the intestine, we focused here on the regulation of the Wnt/β-catenin pathway. We showed that T3-TRα1 controls the expression of the murine β-catenin gene, catnb, by binding on the chromatin of epithelial cells to a functional TRE sequence in intron 1. This effect is parallel to the activation of the Wnt/β-catenin target genes, including type D cyclins and c-myc. This work then provides the first demonstration of the direct control of transcription of the β-catenin gene.
MATERIALS AND METHODS
Animal treatment and tissue preparation.
TRα0/0 (13), TRβ−/− (12), Pax8−/− (28), and the respective wild-type animals have been used in this study. Mice were housed and maintained with approval from the animal experimental committee of the Ecole Normale Supérieure de Lyon (Lyon, France). TH deficiency in pups was induced by feeding the mothers a low-iodine diet supplemented with 0.15% propylthiouracil (PTU; Harlan/Teklad) for 2 weeks. To induce TH deficiency in adult animals, they were fed the low-iodine diet supplemented with 0.15% PTU and 0.05% methimazole (Sigma) in the drinking water for 3 weeks. Hyperthyroidism was induced by intraperitoneal injections of a mixture of T4 and T3 (2.5 mg/kg T4 and 0.25 mg/kg T3 in 100 μl of phosphate-buffered saline) for the indicated length of time. Control animals were fed standard mouse chow. Animals were sacrificed at the indicated ages, and the intestine was quickly removed. The proximal and distal parts of the small intestine were fixed in 10% buffered formalin for immunohistochemistry or frozen in liquid nitrogen and used for RNA and/or protein extraction. Blood was also recovered for serum preparation, and the levels of free T3 and T4 were analyzed by a VIDAS enzyme-linked assay kit (Biomerieux). The data on T3 and T4 measurements are reported in Tables S1 and S2 in the supplemental material.
Primary cell culture of intestine epithelial cells.
Primary intestinal cell cultures were derived from 10-day postnatal mice. After sacrifice, the whole small intestine was removed. The epithelium was isolated as intact organoids by enzymatic dissociation using collagenase type XI (Sigma) and dispase (Boehringer Mannheim) followed by physical disaggregation (8). Organoids were plated in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 2.5% heat-inactivated fetal calf serum (Gibco) depleted of T3 and T4 (45), 20 ng/ml epidermal growth factor (Sigma), and insulin-transferrin-selenium diluted 1/100 (Sigma). Cultures were maintained on 24-multiwell tissue culture dishes. For immunolabeling experiments, coverslips were inserted in the wells. Culture surfaces were coated with type 1 collagen. The purity of the epithelial colonies was analyzed by immunolabeling for specific markers: epithelial cells by anticytokeratins (ICN); fibroblasts by antivimentin (Sigma); smooth muscle cells by anti-smooth muscle actin (Sigma). Cells were analyzed after 5 days of culture. For the analysis of T3 action in vitro, 10−7 M T3 or vehicle alone was added to the culture medium for the indicated length of time. Cycloheximide (15 μg/ml) was added as indicated. For proliferation studies, 10 μM bromodeoxyuridine (BrdU) was added to the culture medium during an overnight incubation.
RNA extraction and analysis.
RNA was extracted from tissues using the QIAGEN RNeasy kit (QIAGEN) and from cell cultures using the Stratagene Absolutely RNA nanoprep kit (Stratagene). To avoid the presence of contaminating DNA in RNA samples, DNase digestion (according to the specific instructions of the manufacturer; QIAGEN or Stratagene) was performed in all preparations. Northern blot analysis was carried using a Hybond N+ membrane (Amersham), and DNA probes for β-catenin, cyclin D1, cyclin D2, c-myc, and hypoxanthine phosphoribosyltransferase (HPRT) were labeled by random priming using [32P]ATP. Real-time quantitative PCR analysis used random hexanucleotide priming and amplification in a Lightcycler apparatus (Bio-Rad Laboratories) using SYBR green PCR master mix according to the manufacturer's instructions (QIAGEN). The primers used are listed in Table S3 in the supplemental material. The data from the PCR were normalized to that of 36B4 levels in each sample. Semiquantitative reverse transcription (RT)-PCR was used to analyze TRα1 expression; HPRT has been used as internal control. The primers were as follows: TRα1, 5′-GGAGATGATTCGCTCACTGCAG and 5′-CGACTTTCATGTGGAGGAAG (product size, 720 bp); HPRT, 5′-GCTGGTGAAAAGGACCTCT and 5′-CACAGGACTAGAACACCTGC (product size, 240 bp).
Cell line culture and transfection.
Cos1 cells were cultured in DMEM supplemented with 5% heat-inactivated fetal calf serum. The different constructs were cloned in the pGL2 basic expression vector (Promega). The wild-type or mutated 36 bp of the catnb gene comprising TRE-int1 (from bp 2140 to 2175 of intron 1) were cloned upstream of a simian virus 40 minimal promoter (29). The cells were transfected using the Exgen transfection reagent (Euromedex). After transfection, cells were maintained in thyroid hormone-depleted serum (45). T3 (10−7 M) or vehicle alone was added to the culture medium 24 h before the end of the culture. Luciferase activity was measured 48 h after transfection using the luciferase dual system (Promega).
Electrophoretic mobility shift assay (EMSA). (i) In vitro protein synthesis.
Full-length cDNA cloned in pSG5 (Stratagene) coding for mouse TRα1, rat TRβ1, and mouse RXRγ were transcribed/translated in vitro using the TNT kit (Promega). Each cDNA was under the control of the T7 promoter.
(ii) EMSA.
The single-strand oligonucleotides were labeled with T4 polynucleotide kinase (Fermentas) in the presence of [γ-32P]ATP. After annealing with the complementary cold oligonucleotides, the probes were purified on a 10% acrylamide gel and the specific bands were eluted overnight at 4°C in Tris-EDTA. Binding reactions were performed for 20 min using the radiolabeled DNA probes (20,000 cpm) and the in vitro-transcribed TR and/or RXR in 10% glycerol, 10 mM HEPES, 30 mM KCl, 4 mM spermidine, 0.1 mM EDTA, 0.25 mM dithiothreitol, 1 mM Na2PO4, and poly(dI-dC) (1.5 μg). Unlabeled specific and nonspecific competitor oligonucleotides were included at the indicated molar excess in the binding reactions. Where indicated, anti-TRα1 antibodies (antibodies raised against a C-terminal peptide and affinity purified with the same peptide) have been included in the reaction mix for 30 min on ice. After binding, reaction samples were loaded on a 5% nondenaturing polyacrylamide gel and electrophoresed for 2 h at 180 V, followed by gel fixation, drying, and exposure to X-ray film.
Western blot and immunolabeling.
Whole-protein extracts were obtained by homogenizing mouse intestine in 5 mM HEPES (pH 7.9), 26% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride. NaCl was added to a final concentration of 300 mM before centrifugation. Supernatant proteins were separated on a 10% acrylamide-bis acrylamide (29:1) gel and transferred to a nitrocellulose membrane (Hybond ECL) before incubation with the first antibody. This was followed by an incubation with secondary anti-rabbit or anti-mouse immunoglobulin G-horseradish peroxidase (Promega). The signal was analyzed by using the enzymatic chemiluminescence detection kit (Amersham).
Antibodies.
We used the following primary antibodies: mouse monoclonal anti-cyclin D2 (Sigma), rabbit anti-β-catenin (Santa Cruz), mouse anti-cyclin D1 (Santa Cruz), rabbit anti-c-myc (a gift from Calame Kathryn, Columbia University College of Physicians and Surgeons, New York), and mouse anti-β-actin (Sigma). Immunohistochemical analysis of β-catenin (Santa Cruz), TRα1 (antibodies raised against a C-terminal peptide and affinity purified with the same peptide), and Ki67 (Novocastra) was performed on 10% buffered formalin-fixed paraffin-embedded tissue sections. Immunolabeling for β-catenin and BrdU (Roche) has been done on 2% paraformaldehyde fixed cell cultures. Secondary fluorescent antibodies were from Jackson Laboratories. Confocal analysis has been performed on a Zeiss Axiovert microscope, with fluorescence microscopy on a Zeiss Axioplan microscope.
ChIP and DNA analysis.
The chromatin immunoprecipitation (ChIP) study was performed on collagenase-dispase-separated epithelial fragments from 3- to 5-day-old mouse intestines. The monolayer of cells was incubated with 1% formaldehyde in DMEM for 5 min at 37°C. The reaction was blocked by the addition of glycine to a final concentration of 125 mM, followed by two washes of cold phosphate-buffered saline (Invitrogen) containing protease inhibitors (Roche). ChIP was conducted using the kit from Upstate according to the manufacturer's instructions. Briefly, 500 ng of sonicated DNA (fragments of 200 to 1,000 bp, by using a Vibra Cell by Sonics and Materials, Inc.) were incubated, respectively, with 10 μl of anti-TRα1, anti-TRβ1 (Affinity Bioreagents), and preimmune sera overnight at 4°C. At the end of the reaction and washing steps, DNA was extracted by phenol-chloroform and suspended in 30 μl of H2O. Four microliters of samples was used for conventional PCR (Taq from Eurogentec). For quantitative PCR we used the Lightcycler apparatus (Bio-Rad Laboratories) and the SYBR green PCR master mix from QIAGEN. The primers are listed in Table S4 in the supplemental material. All of the amplified fragments have been controlled by cloning and sequencing.
RESULTS
TRα gene controls gut epithelial proliferation starting early after birth.
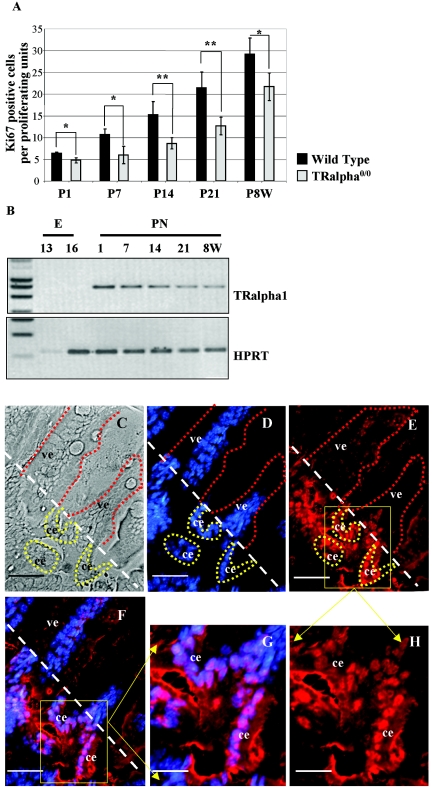
Our previous study indicated the major involvement of the TRα gene in the control of epithelial proliferation in the intestine during weaning time (40, 41). We extended here this observation and analyzed the rate of cell proliferation in the TRα0/0 knockout mice (13) compared to the wild type (WT) throughout fetal and postnatal intestinal development until adulthood (Fig. 1A). In TRα0/0 mutants, we observed a 25% decrease in the number of proliferating cells at postnatal day 1 and in 8-week-old adult animals and a decrease of nearly 50% at 7, 14, and 21 days after birth compared with the respective WT animals. This was observed in both the proximal (Fig. 1A) and distal small intestine (not shown). Looking for the expression of the TRα1 receptor, we could observe that TRα1 mRNA was expressed during this same period (Fig. 1B). Moreover, TRα1 protein, analyzed by an immunohistochemical approach, was specifically present in the crypt cells, as shown for the 14-day-old intestine (Fig. 1C to H; see Fig. S1C to H in the supplemental material).
FIG. 1.
Analysis of crypt cell proliferation and TRα1 expression in the small intestine. (A) Study of cell proliferation during development in the proximal small intestine. Ki67 immunolabeling was performed as previously described (41). The number of Ki67-positive nuclei has been evaluated on well-oriented sections from 4 to 5 different animals per age. Histograms represent means ± standard deviations. Statistical analysis was conducted by using two-tailed Student t test. *, P < 0.05; **, P < 0.001. P, postnatal day; W, weeks. (B) Analysis of TRα1 expression by semiquantitative RT-PCR during intestine development. HPRT was used as an internal control. E, embryo; PN, postnatal. (C to H) TRα1 protein immunolabeling (E and H) on a 2-week-old mouse intestine. In panel C, the phase-contrast picture is shown. All nuclei were stained with Hoechst (D). In panels F and G, the merging of each simple staining is shown. Controls for TRα1 antibody specificity are illustrated in Fig. S4 in the supplemental material. Bars, 15 μm (C to F); 7 μm (G and H). Abbreviations: ve, villus epithelium; ce, crypt epithelium. The white dotted bar defines the limit between the crypt and the villus compartments. Some villi (red dots) and crypts (yellow dots) are highlighted in panels C to E.
Thyroid hormone controls the expression of the Wnt/β-catenin pathway.
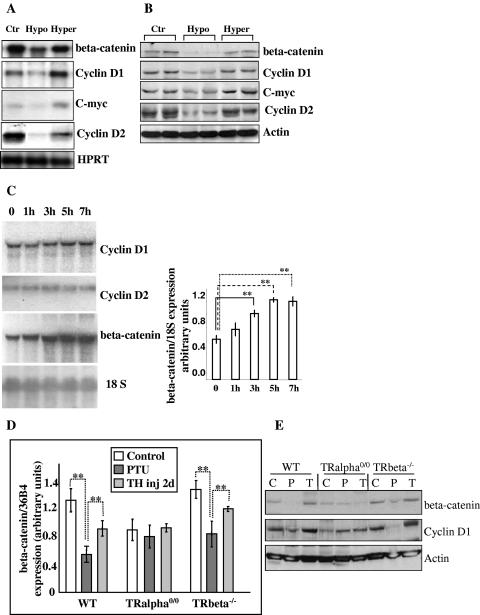
Focusing on 2-week-old animals, we studied the expression of several components of the Wnt pathway, including β-catenin; β-catenin destabilizers adenomatous polyposis coli, glycogen synthase kinase 3β, and axin; β-catenin stabilizer disheveled; β-catenin transcription cofactor TCF4 and its repressors Groucho and Chybby (1, 51). Using RNAs extracted from euthyroid, hypothyroid, and hyperthyroid intestines, we observed that the expression of these genes was unaffected by the TH status (not shown), except for β-catenin. In fact, chemical-induced or congenital hypothyroidism resulted in a decrease of β-catenin mRNA and protein (Fig. 2A and B). TH injections for 2 days resulted in an increase of both mRNA and protein levels. We also analyzed the expression of the downstream targets of this pathway, cyclin D1 and c-myc (1), as well as the indirect target cyclin D2 (20), in animals of different TH status (Fig. 2A and B). All of these genes, expressed mainly in crypt cells where they control the cell proliferation (5, 25, 52), showed a decreased expression at the mRNA and protein levels in hypothyroid conditions and a strong increase after TH injection.
FIG. 2.
Study of TH action in vivo. (A) Representative Northern blot analysis of the indicated mRNAs in 2-week-old intestine of euthyroid (Pax8+/+) (Ctr), congenital hypothyroid (Pax8−/−) (Hypo), and hyperthyroid (Pax8−/− TH-injected) (Hyper) animals. HPRT mRNA was used as an internal control. (B) Representative Western blot analysis of indicated proteins in animals as described for panel A. (C) Kinetic analysis of gene induction by TH evaluated by Northern blotting. PTU-treated animals were injected with TH for the indicated time. 18S RNA was the internal control. Histograms (means ± standard deviations) summarize densitometry analyses from three independent experiments. (D) Quantitative real-time RT-PCR analysis of β-catenin mRNA in intestines of TRα0/0, TRβ −/−, and WT animals displaying different TH status. The picture is representative of two independent experiments, with 3 animals/condition. Histograms represent means ± standard deviations. (E) Representative Western blot analysis of indicated proteins in animals as described for panel D. Statistical analysis was conducted by using two-tailed Student t test. **, P < 0.001. C, control; P, PTU treated; T, TH injected (inj).
We then analyzed the kinetic profile of mRNA induction to clarify the hierarchy of the gene induction. We gave a single pulse of TH to hypothyroid animals and recovered the intestine at different time points: 1 h, 3 h, 5 h, and 7 h after injection. The results are illustrated in Fig. 2C. Cyclins D1 and D2 and c-myc (not shown) mRNA expression were not changed over a period of 7 h after injection (Fig. 2C). On the contrary, β-catenin mRNA started to be increased as early as 1 h after TH injections, being statistically significant after 3 h and reaching a maximum level 5 h after the injection (Fig. 2C). These data indicate that β-catenin induction by TH is an earlier event than with the other studied genes.
We also quantified β-catenin mRNA and protein expression in TRα and TRβ mutant mice having different TH status to evaluate whether TH responsiveness depended on a specific TR pathway (Fig. 2D and E). Wild-type and TRβ −/− animals showed a decreased β-catenin mRNA and protein expression under hypothyroid conditions and an induction after TH treatment for 6 h (not shown) or 2 days. Untreated TRα0/0 mice, which showed a decreased level of β-catenin mRNA compared to wild-type mice under the same conditions, displayed unchanged levels of this mRNA and protein under hypothyroid or hyperthyroid conditions. Similar analysis was performed on wild-type and TRα0/0 adult animals. The results showed that β-catenin gene and protein expression was regulated in a similar manner in the adult and the developing intestine (see Fig. S2A and B in the supplemental material). The parallel analysis of the downstream controlled gene cyclin D1 at the mRNA (not shown) and protein levels (Fig. 2E; see Fig. S2B in the supplemental material) showed a trend of regulation similar to that of β-catenin. It is worth pointing out that both β-catenin and cyclin D1 expression are much more affected by hypothyroidism than by lack of the TRα gene (compare wild-type PTU with TRα0/0). This is probably due to the aporeceptor effect of the nonliganded TRα1 in the absence of T3 (9). Altogether, these data demonstrated that the regulation of β-catenin expression by TH depended specifically on the TRα1 receptor in the developing and the adult intestine.
In vitro analysis of the β-catenin induction by thyroid hormone and TRα gene.
To analyze a homogeneous cell population and to understand whether the responsiveness to TH through the TRα gene is an epithelial cell autonomous process, we used a model of epithelial primary cultures from WT and TR knockout mice. The enzymatic separation between the epithelium and the connective tissue (8) provided a highly pure epithelial cell population (see Fig. S3 in the supplemental material). BrdU incorporation analysis showed that this culture system preserved a population of proliferating cells, presumably epithelial progenitors. However, due to the small size of the epithelial organoids, this model could only be used for immunolabeling and quantitative real-time RT-PCR experiments.
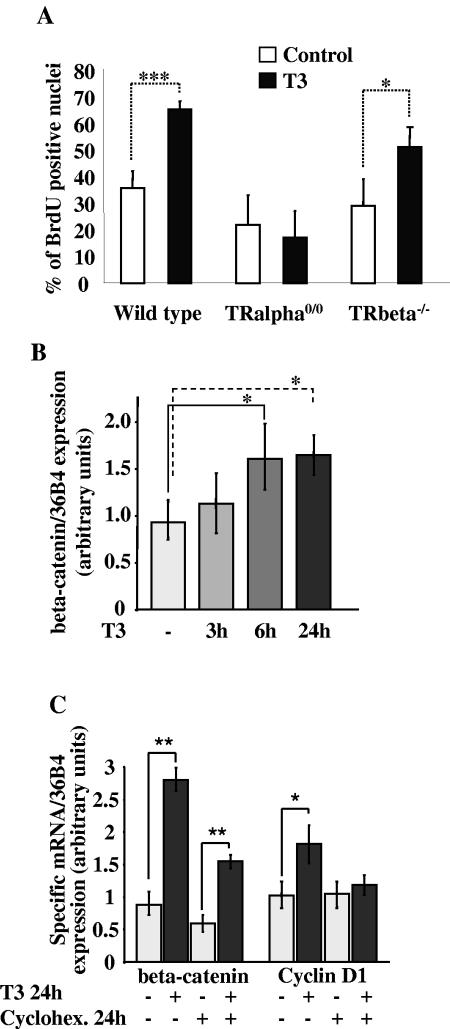
First, we tested whether the cell proliferation in vitro was affected by the addition of T3 in the culture medium (Fig. 3A). Primary cultures established from WT as well as TRβ−/− intestine showed a strong increase in the rate of BrdU-positive cells when maintained for 2 days in the presence of T3 compared with untreated cells. In contrast, TRα0/0-derived cell cultures showed a lower level of proliferating cells than the WT and did not respond to T3 treatment.
FIG. 3.
In vitro study of TH action. (A) Rate of cell proliferation in epithelial primary cultures from WT and TR knockout intestines, after BrdU incorporation and immunolabeling. Total nuclei stained with propidium iodide and BrdU-positive nuclei were counted under the microscope. Histograms (means ± standard deviations) represent results from three independent experiments conducted (each) on 12 1-cm wells. (B) β-Catenin mRNA quantification by real-time RT-PCR, evaluated at different times of T3 treatment. The results (means ± standard deviations) are the summary of those for four independent experiments, each conducted in duplicate. (C) β-Catenin and cyclin D1 mRNA expression in primary cultures maintained during 24 h in the presence (+) or absence (−) of T3 and/or cycloheximide (Cyclohex). Data (means ± standard deviations) summarize the results of two independent experiments conducted in triplicate. Two-tailed Student t test was used. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
The in vitro model was then used to analyze specific gene expression by quantitative real-time RT-PCR. We studied β-catenin expression in vitro in control or T3-treated cells for different lengths of time (Fig. 3B). The mRNA level increased after T3 treatment, reaching statistical significance after 6 h. In vivo studies showed a hierarchy in the induction of gene expression by TH. If the induction of the Wnt/β-catenin target genes by T3-TRα1 depends on the previous induction of the β-catenin, the use of in vitro model could help to answer to this question. For this reason, we treated the cells with cycloheximide and/or T3 for 24 h and analyzed the expression of β-catenin and cyclin D1. In non-cycloheximide-treated cells, we observed an induction of both mRNAs compared to the control (Fig. 3C). The addition of cycloheximide specifically suppressed the induction by T3 of cyclin D1 expression. On the contrary, cycloheximide had no effect on β-catenin up regulation by T3 (Fig. 3C). The small decrease of gene expression in the cycloheximide-treated cells is probably due to a generalized action of the drug. These data demonstrate that the positive regulation of the β-catenin gene expression does not need protein synthesis, strongly suggesting a direct effect on β-catenin but not on the cyclin D1 gene.
Characterization of a functional TRE in intron 1 of the β-catenin gene: in vitro and in vivo studies.
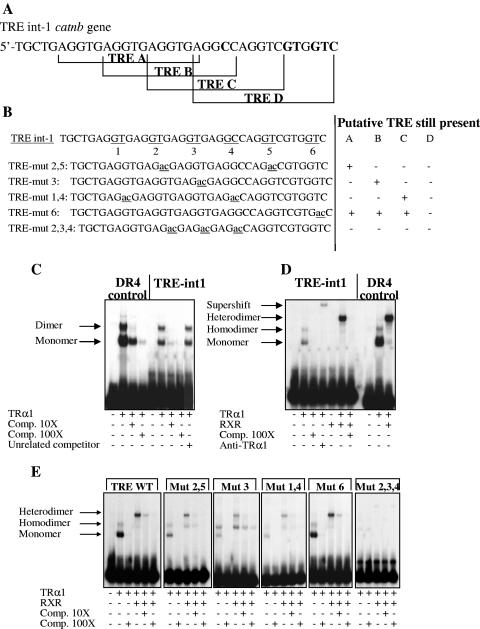
The data on the regulation of β-catenin gene by TH and TRα1, in vivo and in vitro, suggested that this gene may be a direct target of the TRα1 receptor. Looking in silico for prediction of TRE sequences in the mouse β-catenin gene catnb, we found a sequence of 36 bp (referred as TRE-int1), spanning from nucleotide 2140 of the first intron and containing four putative TRE sites (Fig. 4A). While the TRE-A is a perfect DR4 (6), the sites TRE-B, TRE-C, and TRE-D show degenerate DR4 structures. To evaluate whether the TRα1 receptor binds in vitro to these TREs, we performed EMSAs. Compared to a synthetic positive control DR4 (29), the TRE-int1 has the same ability to bind to TRα1 as a monomer, homodimer, and TRα1/RXR heterodimer (Fig. 4C and D). These complexes were supershifted after incubation with an antibody against TRα1 (Fig. 4D). We also tested by EMSA different mutant TRE-int1 sequences (Fig. 4B). Mutant 2,5, mutant 6, and to a lesser extent, mutant 3 retained their ability to bind to TRα1 as a monomer, homodimer, and heterodimer (Fig. 4E). Mutant 1,4 had a lower ability, and mutant 2,3,4 was unable to bind to TRα1.
FIG. 4.
Molecular analysis of the thyroid hormone responsive element present in the catnb gene. (A) Structure of the four putative DR4 found in intron 1. Divergences from canonical DR4 are indicated in boldface type. (B) WT TREint-1, showing the GT sequences (1 to 6) substituted in the different TRE mutants. (C to E) EMSA analysis using the labeled probes indicated and in vitro-transcribed/translated proteins indicated. The specificity of the binding was assessed by adding specific or nonrelated cold sequences at the indicated molar excess (Comp. 10× or Comp. 100×). +, present; −, absent.
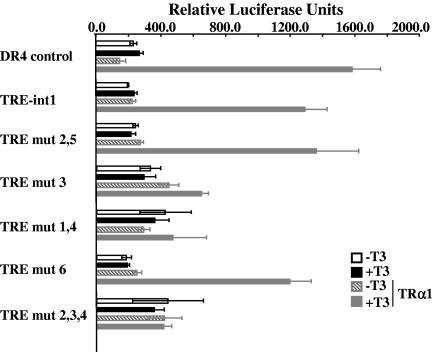
Next, we analyzed the enhancer properties of the β-catenin TREs in transient-transfection experiments. We cloned the 36-bp TRE-int1 sequence upstream of a minimal simian virus 40 promoter (29) in a luciferase reporter vector and then transfected it in Cos1 cells (Fig. 5). The expression of the luciferase was analyzed in the presence or absence of either TRα1 expression vector and/or T3. As Cos1 cells contain low levels of endogenous TRα1, induction of the DR4 positive control by T3 was observed only in cultures cotransfected with the TRα1 expression vector. A similar response was observed with the TRE-int1 reporter, showing that it is functionally responsive to T3. When we compared the different TRE-int1 mutant constructs, it was clear that only those preserving the TRE-A, alone or in combination with TRE-B and C, displayed a responsiveness to T3 similar to that of the TRE-int1. Altogether, these data strongly suggested that TRE-A is the functional binding site for TRα1.
FIG. 5.
Transient-transfection analysis in Cos1 cells. Two hundred nanograms of the luciferase expression vector was transfected alone or in combination with 200 ng of pSG5-hTRα1 expression vector. Cells were treated or not with 10−7 M T3 for 16 h. Bars represent means ± standard deviations (n = 6).
To check whether the TRE-int1 is a binding site for TRα1 in vivo, we investigated the binding by in vivo ChIP assay. We performed these experiments on collagenase-dispase-isolated fragments of the intestinal epithelium, freshly prepared from newborn animals. Figure 6 summarizes the set up and the results of these experiments. The sonicated chromatin has been incubated with anti-TRα1, anti-TRβ1, or preimmune sera. The precipitated DNA from these different experimental conditions and in the starting inputs was then analyzed by conventional and real-time PCR to reveal the presence of specific DNA templates. All of the amplified products have been controlled by cloning and sequencing (not shown). Interestingly, using the Catnb1 and Catnb2 primers, which amplify DNA fragments of the catnb gene, including the TRE-int1 (Fig. 6A), we showed a specific band only in the samples incubated with the anti-TRα1 antibody (Fig. 6B, lane b). A band of the same size was obviously present in the starting input samples (Fig. 6B, lane d). To verify the specificity of this result, we performed a PCR analysis using the Catnb3 and Catnb4 primers, which amplify fragments of the intron 1 outside the TRE (Fig. 6A). Moreover, we used primers specific to promoters of the phosphoriboprotein gene 36B4 and of the villin gene, for which no regulation by TH has been described. Using these oligonucleotides, we only amplified DNA fragments in the starting inputs (Fig. 6B, lanes d), indicating that the anti-TRα1 antibody does not bind the chromatin aspecifically. To quantify the amount of the DNA precipitated by the TRα1 antibodies, we also performed real-time PCR. Using the Catnb1 (Fig. 6C) and Catnb2 (not shown) primers, we amplified specific DNA fragments. The amounts are expressed as percentages of the starting inputs. No specific amplification could be observed in the samples incubated with the anti-TRβ1 or preimmune sera or in the different samples when we used the Catnb3 (Fig. 6C), Catnb4, and p36B4 (not shown) primers. These data clearly demonstrated that, in intestinal epithelial cells in vivo, TRα1 binds to the β-catenin gene at the level of the TRE-int1.
FIG. 6.
In vivo ChIP. (A) Scheme of intron 1 of the catnb gene displaying the different primers used for this study. S (sense) and AS (antisense) numbers refer to oligonucleotide primers of the respective Catnb-amplified fragment numbers. E1 and E2 refer to exons 1 and 2, respectively. The arrow upstream of E1 indicates the promoter of the gene. Note that Catnb1 and Catnb2 primers amplify DNA fragments comprising the TRE. Catnb3 and Catnb4 were used as negative controls, as the amplified fragments are, respectively, 500 bp and 3,000 bp away from the TRE. (B) Study by conventional PCR of the DNA purified from the different samples before and after ChIP. The picture is representative of the results from two independent experiments. On the right part of each panel, the fragment amplified on the catnb gene or on the promoters of 36B4 and villin genes is indicated: a, preimmune serum; b, anti-TRα1; c, anti-TRβ1; d, starting input; e, negative control for PCR mix. (C) Quantitative real-time PCR study of the purified DNA before and after ChIP. The amount of the indicated products is illustrated as a percentage of that measured in the starting inputs. The picture is representative of the results of two independent experiments.
DISCUSSION
In this paper and in previous reports, we pointed out that T3-activated-TRα1 receptor controls the proliferation of the intestinal epithelial cells in vivo and in vitro. In an attempt to identify the molecular basis of this control, we focused here on the analysis of the Wnt/β-catenin pathway, since it plays a central role in the control of normal and pathological cell proliferation in the gut (42). Interestingly, we observed that the TRα1 receptor directly controls the transcription of the β-catenin gene, the central actor of this pathway. In fact, in vivo and in vitro models treated with TH displayed an increase in β-catenin mRNA and protein expression. This was parallel to the up-regulation of cyclins D1 and D2 and c-myc, well-known proliferation controlling genes (5, 25, 52) and targets of the Wnt/β-catenin pathway (1, 20). Several findings support the assertion that the β-catenin gene, but not cyclin D or c-myc, is directly regulated by TH. First, induction of the β-catenin mRNA in vivo and in vitro is achieved after a short time of treatment by TH. Second, the cycloheximide addition to the culture medium does not prevent the up-regulation of the β-catenin mRNA in vitro by T3, as it is the case for cyclin D1 mRNA. Third, TH injections to TRα0/0 animals, which are unable to stimulate β-catenin expression, also fail to increase the cyclin D1 mRNA compared to the WT animals (data not shown). Finally, we also provided the experimental proof that in epithelial cells of the intestine in vivo, TRα1 is fixed on the TRE-int1 present in the catnb gene. This is the first report of in vivo ChIP analysis which shows the occupancy of a TRE by a TR receptor in mammals. A binding of TRE by TRs has already been reported for Xenopus laevis target genes (44).
The regulation of the transcription of the β-catenin gene by T3 is likely to go through the TRE-int1. In fact, it is the unique responsive element found within the promoter environment. Moreover, its intronic location is not surprising, since several reports described TREs located downstream of the promoters (7, 38, 46). We also showed that the TRE-int1 is composed of several TREs organized in tandem, reminiscent of the TREs present in the rat growth hormone gene (37).
Despite the well-described posttranslational control of the β-catenin, very little is known at the gene transcription level. A recent paper described the cloning and analysis of the rat and human β-catenin regulatory regions of this gene (23). Interestingly, several AP1 binding sites and one TCF binding site have been described, suggesting that several putative factors may influence the transcription of this gene. However, no physiological functions for these regulatory regions have been reported until now. We showed here that the TRE in intron 1 of the catnb gene is functional in vivo and in vitro.
β-Catenin is a central actor in gut epithelial cell homeostasis, and its subcellular localization defines specific functions in cell adhesion (membrane bound) or in target gene activation (nuclear presence) (36, 52). We reported in our study that TH deprivation or supplementation in animals and in cells affects β-catenin gene and protein expression. More specifically, TH treatment allows an increase of its levels. The availability of free β-catenin, which migrates into the nuclei, is controlled by several mechanisms involving stabilizing and destabilizing phosphorylations as well as destabilizing dephosphorylations (36). In TH-stimulated models, we observed an increase of β-catenin-positive nuclei (not shown), strongly suggesting that this treatment positively affects its stabilization. However, the exact mechanisms responsible for this remain to be clarified and will be the subject of future investigations.
In the nucleus, β-catenin activates the transcription of target genes, as it binds to the Tcf/Lef family of transcription factors (35). Here we show that some of its targets, activators of cell proliferation, are positively regulated by TH in vivo and in vitro. This strongly suggests that the increase of cell proliferation induced by TH may be dependent on the activation of β-catenin expression. Is the increase of the β-catenin expression per se sufficient to induce cell proliferation? This is a crucial question. Several reports indicated that increasing the level of β-catenin leads to increased cell proliferation in various systems, such as the intestine (47, 55), liver (32, 34), and vestibular cells (19). This strongly supports our own data showing that the increase of β-catenin expression is one key molecular event related to the activation of cell proliferation. However, it is worth pointing out that a negative control by TH of the Wnt pathway was described in an in vitro model of pituitary cells (33). In this model, the positive action of the TH on cell proliferation was parallel to a decrease of β-catenin expression. However, there was no indication of a direct negative control of the β-catenin gene by TRs in these cells. The different model systems analyzed and differences in the cellular and molecular microenvironment may account for the discrepancy with our results. It is worth pointing out that our results are consistent with the well-established fact that the Wnt/β-catenin pathway is a positive regulator of the intestine cell proliferation.
Our observations suggested that TH signaling in the intestine is essential throughout intestine development and in adulthood. However, a major role is played during postnatal development, when an extensive growth takes place (39). Indeed, this period also corresponds in the mouse to a surge of TH, which declines starting from the third week to reach a steady-state adult level (14). According to this, TRα knockout mice show more intestinal impairment during the first 3 weeks after birth than in adulthood. Moreover, the absence of TH production during early postnatal development, as in the congenital hypothyroid Pax8−/− mice (28), has dramatic effects on intestine development, much more so than in TRα mutants (9). This can be explained by the aporeceptor function of TRα1 in absence of TH, acting as a transcription repressor of target genes (9, 26). In this light, the repression of β-catenin and of its downstream targets observed in hypothyroid animals (both congenital and chemical induced), may help explain some traits of the intestine phenotype. Indeed, removal of the TRα1 receptor in Pax8−/− animals was previously reported to induce a recovery of intestine development (9).
Together, these data indicated that T3-activated TRα1 plays an essential role in postnatal intestine development as well as in adult homeostasis. Our present data show that the action on the β-catenin gene control is one of the molecular processes involved.
Until now few studies pointed to a function of TH and TR in gastrointestinal physiopathology (11, 15, 17, 27, 31). Some data showed a correlation between altered levels of TH and the incidence of breast and colon cancer in humans (43). On the other hand, it has been reported that hyperthyroxinemia can influence the rate of colon cancer in an experimental model of carcinogenesis in rats (24). Finally, in some cases, the absence (30) or the mutation (53) of the TRs have been associated with gastrointestinal tumors. In our study, we show that TH and TRα1 receptor control epithelial cell proliferation and the expression of components of the Wnt pathway. As β-catenin and some of its target genes are involved in intestinal tumorigenesis, our data open new perspectives in the function of the TRα1 receptor as a potential tumor inducer, as is the case for other nuclear receptors (2).
Supplementary Material
Acknowledgments
We thank the animal (PBES) and microscopy (PLATIM) facilities of the IFR 128 Biosciences. We also thank the ANIGENE platform of Rhône-Alpes Génopole. We gratefully acknowledge Nadine Aguilera for animal handling. We thank N. Davidson, K. Gauthier, and B. Pain for critical reading of the manuscript and O. Bakker for help in immunolabeling for TRα1.We are grateful to A. Rezza for participation in experiments with adult animals.
This work was supported by a grant from the Ligue Nationale contre le Cancer to J.S. (Equipe Labellisée). E.K. was a fellow of the Ligue Nationale contre le Cancer.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Akiyama, T. 2000. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 11:273-282. [DOI] [PubMed] [Google Scholar]
- 2.Altucci, L., and H. Gronemeyer. 2001. Nuclear receptors in cell life and death. Trends Endocrinol. Metab. 12:460-468. [DOI] [PubMed] [Google Scholar]
- 3.Burgess, A. W. 1998. Growth control mechanisms in normal and transformed intestinal cells. Philos. Trans. R. Soc. Lond. B 353:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekaran, C., C. M. Coopersmith, and J. I. Gordon. 1996. Use of normal and transgenic mice to examine the relationship between terminal differentiation of intestinal epithelial cells and accumulation of their cell cycle regulators. J. Biol. Chem. 271:28414-28421. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, S. Y. 2000. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 7.Ciana, P., G. G. Braliou, F. G. Demay, M. von Lindern, D. Barettino, H. Beug, and H. G. Stunnenberg. 1998. Leukemic transformation by the v-ErbA oncoprotein entails constitutive binding to and repression of an erythroid enhancer in vivo. EMBO J. 17:7382-7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, G. S., N. Flint, A. S. Somers, B. Eyden, and C. S. Potten. 1992. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 101:219-231. [DOI] [PubMed] [Google Scholar]
- 9.Flamant, F., A. L. Poguet, M. Plateroti, O. Chassande, K. Gauthier, N. Streichenberger, A. Mansouri, and J. Samarut. 2002. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol. Endocrinol. 16:24-32. [DOI] [PubMed] [Google Scholar]
- 10.Flamant, F., and J. Samarut. 2003. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol. Metab. 14:85-90. [DOI] [PubMed] [Google Scholar]
- 11.Freedberg, A., and M. Halmolsky. 1974. Effects of thyroid hormone on certain non endocrine organ systems, p 435-468. In M. Greer and E. Solomon (ed.), Handbook of physiology-endocrinology-thyroid. American Physiological Society, Washington, D.C.
- 12.Gauthier, K., O. Chassande, M. Plateroti, J. P. Roux, C. Legrand, B. Pain, B. Rousset, R. Weiss, J. Trouillas, and J. Samarut. 1999. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 18:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier, K., M. Plateroti, C. B. Harvey, G. R. Williams, R. E. Weiss, S. Refetoff, J. F. Willott, V. Sundin, J. P. Roux, L. Malaval, M. Hara, J. Samarut, and O. Chassande. 2001. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol. Cell. Biol. 21:4748-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadj-Sahraoui, N., I. Seugnet, M. T. Ghorbel, and B. Demeneix. 2000. Hypothyroidism prolongs mitotic activity in the post-natal mouse brain. Neurosci. Lett. 280:79-82. [DOI] [PubMed] [Google Scholar]
- 15.Henning, S., D. Rubin, and J. Shulman. 1994. Ontogeny of the intestinal mucosa, p. 571-601. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract, 3rd ed. Raven Press, New York, N.Y.
- 16.Hermiston, M., T. Simon, M. Crossman, and J. I. Gordon. 1994. Model systems for studying cell fate specification and differentiation in the gut epithelium, p. 521-570. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract, 3rd ed. Raven Press, New York, N.Y.
- 17.Jumarie, C., and C. Malo. 1994. Alkaline phosphatase and peptidase activities in Caco-2 cells: differential response to triiodothyronine. In Vitro Cell. Dev. Biol. Anim. 30A:753-760. [DOI] [PubMed] [Google Scholar]
- 18.Kedinger, M. 1994. Growth and development of intestinal mucosa, p. 1-31. In F. C. Campbell (ed.), Small bowel enterocyte culture and transplantation. R. G. Landes Company, Austin, Tex.
- 19.Kim, T. S., T. Nakagawa, J. E. Lee, K. Fujino, F. Iguchi, T. Endo, Y. Naito, K. Omori, P. P. Lefebvre, and J. Ito. 2004. Induction of cell proliferation and beta-catenin expression in rat utricles in vitro. Acta Otolaryngol. Suppl. 551:22-25. [DOI] [PubMed] [Google Scholar]
- 20.Kioussi, C., P. Briata, S. H. Baek, D. W. Rose, N. S. Hamblet, T. Herman, K. A. Ohgi, C. Lin, A. Gleiberman, J. Wang, V. Brault, P. Ruiz-Lozano, H. D. Nguyen, R. Kemler, C. K. Glass, A. Wynshaw-Boris, and M. G. Rosenfeld. 2002. Identification of a Wnt/Dvl/beta-Catenin→Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111:673-685. [DOI] [PubMed] [Google Scholar]
- 21.Laudet, V., C. Hanni, J. Coll, F. Catzeflis, and D. Stehelin. 1992. Evolution of the nuclear receptor gene superfamily. EMBO J. 11:1003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar, M. A. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 14:184-193. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., W. M. Dashwood, X. Zhong, M. Al-Fageeh, and R. H. Dashwood. 2004. Cloning of the rat beta-catenin gene (Ctnnb1) promoter and its functional analysis compared with the Catnb and CTNNB1 promoters. Genomics 83:231-242. [DOI] [PubMed] [Google Scholar]
- 24.Lishi, H., M. Tatsuta, M. Baba, S. Okuda, and H. Taniguchi. 1992. Enhancement by thyroxine of experimental carcinogenesis induced in rat colon by azoxymethane. Int. J. Cancer 50:974-976. [DOI] [PubMed] [Google Scholar]
- 25.Lynch, J., E. R. Suh, D. G. Silberg, S. Rulyak, N. Blanchard, and P. G. Traber. 2000. The caudal-related homeodomain protein Cdx1 inhibits proliferation of intestinal epithelial cells by down-regulation of D-type cyclins. J. Biol. Chem. 275:4499-4506. [DOI] [PubMed] [Google Scholar]
- 26.Mai, W., M. F. Janier, N. Allioli, L. Quignodon, T. Chuzel, F. Flamant, and J. Samarut. 2004. Thyroid hormone receptor alpha is a molecular switch of cardiac function between fetal and postnatal life. Proc. Natl. Acad. Sci. USA 101:10332-10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malo, M. S., W. Zhang, F. Alkhoury, P. Pushpakaran, M. A. Abedrapo, M. Mozumder, E. Fleming, A. Siddique, J. W. Henderson, and R. A. Hodin. 2004. Thyroid hormone positively regulates the enterocyte differentiation marker intestinal alkaline phosphatase gene via an atypical response element. Mol. Endocrinol. 18:1941-1962. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri, A., K. Chowdhury, and P. Gruss. 1998. Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet. 19:87-90. [DOI] [PubMed] [Google Scholar]
- 29.Marchand, O., R. Safi, H. Escriva, E. Van Rompaey, P. Prunet, and V. Laudet. 2001. Molecular cloning and characterization of thyroid hormone receptors in teleost fish. J. Mol. Endocrinol. 26:51-65. [DOI] [PubMed] [Google Scholar]
- 30.Markowitz, S., M. Haut, T. Stellato, C. Gerbic, and K. Molkentin. 1989. Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J. Clin. Investig. 84:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matosin-Matekalo, M., J. E. Mesonero, T. J. Laroche, M. Lacasa, and E. Brot-Laroche. 1999. Glucose and thyroid hormone co-regulate the expression of the intestinal fructose transporter GLUT5. Biochem. J. 339:233-239. [PMC free article] [PubMed] [Google Scholar]
- 32.Micsenyi, A., X. Tan, T. Sneddon, J. H. Luo, G. K. Michalopoulos, and S. P. Monga. 2004. Beta-catenin is temporally regulated during normal liver development. Gastroenterology 126:1134-1146. [DOI] [PubMed] [Google Scholar]
- 33.Miller, L. D., K. S. Park, Q. M. Guo, N. W. Alkharouf, R. L. Malek, N. H. Lee, E. T. Liu, and S. Y. Cheng. 2001. Silencing of Wnt signaling and activation of multiple metabolic pathways in response to thyroid hormone-stimulated cell proliferation. Mol. Cell. Biol. 21:6626-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monga, S. P., H. K. Monga, X. Tan, K. Mule, P. Pediaditakis, and G. K. Michalopoulos. 2003. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology 124:202-216. [DOI] [PubMed] [Google Scholar]
- 35.Moon, R. T., A. D. Kohn, G. V. De Ferrari, and A. Kaykas. 2004. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 5:691-701. [DOI] [PubMed] [Google Scholar]
- 36.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman, M. F., T. N. Lavin, J. D. Baxter, and B. L. West. 1989. The rat growth hormone gene contains multiple thyroid response elements. J. Biol. Chem. 264:12063-12073. [PubMed] [Google Scholar]
- 38.Pain, B., F. Melet, P. Jurdic, and J. Samarut. 1990. The carbonic anhydrase II gene, a gene regulated by thyroid hormone and erythropoietin, is repressed by the v-erbA oncogene in erythrocytic cells. New Biol. 2:284-294. [PubMed] [Google Scholar]
- 39.Plateroti, M., C. Angelin-Duclos, F. Flamant, and J. Samarut. 2004. Tissues specific action of thyroid hormones; insight from knock-out animal models, p. 13-35. In P. Beck-Peccoz (ed.), Syndromes of hormone resistance on the hypothalamic-pituitary-thyroid axis. Kluwer Academic Publishers, Milano, Italy.
- 40.Plateroti, M., O. Chassande, A. Fraichard, K. Gauthier, J. N. Freund, J. Samarut, and M. Kedinger. 1999. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 116:1367-1378. [DOI] [PubMed] [Google Scholar]
- 41.Plateroti, M., K. Gauthier, C. Domon-Dell, J. N. Freund, J. Samarut, and O. Chassande. 2001. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol. Cell. Biol. 21:4761-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 43.Rose, D. P., and T. E. Davis. 1981. Plasma thyronine levels in carcinoma of the breast and colon. Arch. Intern. Med. 141:1161-1164. [PubMed] [Google Scholar]
- 44.Sachs, L. M., and Y. B. Shi. 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 97:13138-13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuels, H. H., F. Stanley, and J. Casanova. 1979. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80-85. [DOI] [PubMed] [Google Scholar]
- 46.Sap, J., L. de Magistris, H. Stunnenberg, and B. Vennstrom. 1990. A major thyroid hormone response element in the third intron of the rat growth hormone gene. EMBO J. 9:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellin, J. H., S. Umar, J. Xiao, and A. P. Morris. 2001. Increased beta-catenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res. 61:2899-2906. [PubMed] [Google Scholar]
- 48.Shi, Y. B., J. Wong, M. Puzianowska-Kuznicka, and M. A. Stolow. 1996. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thyroid hormone and its receptors. Bioessays 18:391-399. [DOI] [PubMed] [Google Scholar]
- 49.Stappenbeck, T. S., M. H. Wong, J. R. Saam, I. U. Mysorekar, and J. I. Gordon. 1998. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr. Opin. Cell Biol. 10:702-709. [DOI] [PubMed] [Google Scholar]
- 50.Taipale, J., and P. A. Beachy. 2001. The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349-354. [DOI] [PubMed] [Google Scholar]
- 51.Takemaru, K., S. Yamaguchi, Y. S. Lee, Y. Zhang, R. W. Carthew, and R. T. Moon. 2003. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422:905-909. [DOI] [PubMed] [Google Scholar]
- 52.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241-250. [DOI] [PubMed] [Google Scholar]
- 53.Wang, C. S., K. H. Lin, and Y. C. Hsu. 2002. Alterations of thyroid hormone receptor alpha gene: frequency and association with Nm23 protein expression and metastasis in gastric cancer. Cancer Lett. 175:121-127. [DOI] [PubMed] [Google Scholar]
- 54.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 55.Wong, M. H., B. Rubinfeld, and J. I. Gordon. 1998. Effects of forced expression of an NH2-terminal truncated beta-Catenin on mouse intestinal epithelial homeostasis. J. Cell Biol. 141:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.