Abstract
Surfactant protein A (SP-A) is important for immune defense within the alveolus. Cyclic AMP (cAMP) stimulation of SP-A expression in lung type II cells is O2 dependent and mediated by increased phosphorylation and binding of thyroid transcription factor 1 (TTF-1) to an upstream response element (TTF-1-binding element [TBE]). Interleukin-1 (IL-1) stimulation of SP-A expression is mediated by NF-κB (p65/p50) activation and increased binding to the TBE. In this study, we found that O2 also was permissive for IL-1 induction of SP-A expression and for cAMP and IL-1 stimulation of type II cell nuclear protein binding to the TBE. Using chromatin immunoprecipitation, we observed that when type II cells were cultured in 20% O2, cAMP and IL-1 stimulated the recruitment of TTF-1, p65, CBP, and steroid receptor coactivator 1 to the TBE region of the SP-A promoter and increased local acetylation of histone H3; these effects were prevented by hypoxia. Hypoxia markedly reduced global levels of CBP and acetylated histone H3 and increased the expression of histone deacetylases. Furthermore, hypoxia caused a global increase in histone H3 dimethylated on Lys9 and increased the association of dimethyl histone H3 with the SP-A promoter. These results, together with findings that the histone deacetylase inhibitor trichostatin A and the methyltransferase inhibitor 5′-deoxy(5′-methylthio)adenosine markedly enhanced SP-A expression in lung type II cells, suggest that increased O2 availability to type II cells late in gestation causes epigenetic changes that permit access of TTF-1 and NF-κB to the SP-A promoter. The binding of these transcription factors facilitates the recruitment of coactivators, resulting in the further opening of the chromatin structure and activation of SP-A transcription.
To define mechanisms involved in lung cell-specific, developmental, and hormonal regulation of pulmonary surfactant synthesis, we have focused on surfactant protein A (SP-A), the major surfactant protein, which is lung specific and synthesized in type II cells. SP-A, a member of the collectin subfamily of C-type lectins, plays an important role in immune defense within the lung alveolus by binding to a variety of bacterial, viral, and fungal pathogens to facilitate their uptake by alveolar macrophages and to enhance macrophage migration and cytokine production (see reference 53 for a review). SP-A is an excellent marker of type II cell differentiation and surfactant synthesis in the fetal lung, since it is developmentally regulated with surfactant phospholipid synthesis (see reference 36 for a review). Recently, we obtained compelling evidence that SP-A secreted by the fetal lung into amniotic fluid acts as a hormone that stimulates the migration of amniotic fluid macrophages to the uterus, where they initiate an inflammatory response leading to parturition (13).
In studies using midgestation human fetal lung explants cultured in a 20% O2 environment, we found that type II cell differentiation and SP-A gene expression were enhanced by hormones and factors that increase cyclic AMP (cAMP) (43). Interestingly, cAMP stimulation of type II cell differentiation and SP-A expression are dependent upon a critical atmospheric O2 tension (1). When fetal lung explants were cultured in a 1 to 2% O2-containing environment, type II cells failed to differentiate, and no stimulatory effects of cAMP on SP-A expression were apparent. These findings led us to postulate that increased vascularization of the fetal lung during the third trimester of gestation and enhanced O2 availability to type II cell precursors may facilitate cAMP induction of type II cell differentiation and surfactant protein gene expression (1).
To define genomic regions and response elements involved in lung cell-specific, developmental, and hormonal regulation of SP-A expression, we have utilized transgenic mice and transfected type II cells. In transgenic mice, we found that as little as ∼400 bp of DNA flanking the 5′ end of the rabbit SP-A gene directed appropriate lung cell-specific and developmental regulation of expression (3). This region contains four response elements that are individually required for basal and cAMP stimulation of SP-A promoter activity in transfected type II cells (2, 17, 32, 39, 56, 57). The finding that mutagenesis of any one of these elements markedly reduced basal and cAMP induction of SP-A promoter activity suggests a cooperative interaction of transcription factors binding to these sites. One of these response elements, termed thyroid transcription factor 1 (TTF-1)-binding element (TBE), at −170 bp binds the homeodomain factor, thyroid transcription factor 1 (TTF-1/Nkx2.1/T/EBP) (31, 32). Cyclic AMP enhances the binding of type II cell nuclear proteins to the TBE as well as TTF-1 phosphorylation, acetylation, and transcriptional activity (31, 55). TTF-1 also regulates the expression of the mouse SP-A gene (9) as well as genes encoding SP-B (8) and SP-C (25). TTF-1 is expressed from the earliest stages of lung development (30), where it plays an essential role in branching morphogenesis (26). On the other hand, SP-A and the other surfactant protein genes are developmentally induced at later gestational time points (36). We therefore postulate that these temporally distinct actions of TTF-1 may be mediated by selective changes in TTF-1 posttranslational modification and interaction with other critical transcription factors and coactivators.
In addition to the observed effects of cAMP, we found that treatment of human fetal lung type II cells with the cytokines interleukin-1α (IL-1α) and IL-1β increased SP-A expression and binding of type II cell nuclear proteins to the TBE; IL-1 also had an additive stimulatory effect with cAMP (20). In characterizing the TBE, we noted that it contains an inverted binding site for the transcription factor NF-κB, which is activated by cytokines and protein kinase A (PKA) (7, 58). In its inactive state, NF-κB, a heterodimer of p50 and p65, exists in the cytoplasm in a complex with “inhibitor of κB” (IκB). Exposure of cells to cytokines causes phosphorylation and proteolytic degradation of IκB, with subsequent activation and translocation of the p50/p65 heterodimer to the nucleus, where it binds to response elements in target genes (7). Reactive oxygen species (ROS) serve a permissive role in cytokine activation of NF-κB by facilitating cytokine-mediated serine phosphorylation of IκB by IκB kinase, with consequent IκB degradation via the proteasome pathway (33). We observed that NF-κB p50 and p65 interact with TTF-1 at the TBE and act synergistically with TTF-1 to increase SP-A promoter activity (20). Although direct binding of NF-κB to the TBE has not been established, we observed that TTF-1 interacts with NF-κB p65 in vivo (20). Furthermore, antioxidant NF-κB inhibitors as well as dominant-positive forms of IκB (which block NF-κB activation) reduced type II cell nuclear protein binding to the TBE and blocked inductive effects of cAMP and IL-1 on SP-A expression (20). These findings suggest that NF-κB acts together with TTF-1 at the TBE to activate SP-A gene expression.
The coactivators CREB-binding protein (CBP) and steroid receptor coactivator 1 (SRC-1) synergistically interact with TTF-1 at the TBE to increase SP-A promoter activity (55). TTF-1 binds to CBP and SRC-1 in vitro (55). NF-κB p65 also interacts with CBP and its homologue, p300 (19, 59); phosphorylation of p65 by PKA increases transcriptional activity by enhancing its association with CBP (59). On the other hand, SRC-1 interacts with p50 to enhance NF-κB-mediated transcription (40). In light of these findings, we propose that TTF-1 and NF-κB proteins may activate SP-A gene transcription by forming a transcriptional activation complex at the TBE that is stabilized by multivalent interactions with coactivators. In the present study, we tested the hypothesis that increased O2 availability plays a permissive role in cAMP and cytokine induction of SP-A expression by facilitating the binding of TTF-1 and NF-κB to the TBE as well as in promoting the recruitment of essential coregulators, which in turn mediate changes in histone posttranslational modifications and the opening of the chromatin structure.
MATERIALS AND METHODS
Preparation and culture of human fetal lung explants and type II cells.
Midgestation human fetal lung tissues were obtained from Advanced Bioscience Resources, Inc., in accordance with the Donors Anatomical Gift Act of the State of Texas; protocols were approved by the Human Research Review Committee of the University of Texas Southwestern Medical Center at Dallas. Type II cells were isolated from cultured midgestation human fetal lung explants, as described previously (4). Briefly, lung explants were maintained in organ culture in serum-free Waymouth's MB752/1 medium (Life Technologies, Inc.) in the presence of dibutyryl cAMP (Bt2cAMP) to promote type II cell differentiation (47). After 3 days of culture, tissues were digested with collagenase; isolated cells were treated with DEAE-dextran and plated at a density of 5 × 106 to 9 × 106 cells/60-mm dish. The cells were maintained overnight in Waymouth's medium containing 10% fetal bovine serum and then placed in serum-free Waymouth's medium (control medium) for 24 h at 37°C in a humidified atmosphere of 95% air and 5% CO2 (20% O2) or placed in a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA) in an atmosphere containing 2% O2, 93% N2, and 5% CO2, followed by incubation in these environments for up to 2 days in control medium in the absence or presence of Bt2cAMP (1 mM) (Roche/BMB), IL-1α (10 ng/ml) (Sigma), or Bt2cAMP and IL-1α in combination. In other experiments, type II cells were cultured in a 20% O2 environment for 24 h in the absence or presence of the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) (50 nM; Sigma) (14) or the protein methylation inhibitor 5′-deoxy-5′-(methylthio)adenosine (d-MTA) (300 μM; Sigma) (48). TSA and d-MTA were dissolved in dimethyl sulfoxide (DMSO), and an equivalent amount of DMSO vehicle was added to the untreated dishes. Bt2cAMP was then added to half of the dishes, and the incubation was continued in the absence or presence of these inhibitors for an additional 24 h.
Electrophoretic mobility shift assays (EMSAs).
Nuclear extracts were prepared from lung type II cells as described previously (57). Protein concentrations were determined by a modified Bradford assay (Bio-Rad). Double-stranded oligonucleotides corresponding to the TBE (underlined) and flanking sequences (5′-GTGCTCCCCTCAAGGGTCCT-3′) (Integrated DNA Technologies, Inc.) were end labeled using [γ32P]ATP (Perkin-Elmer, NEN) and used as probes. Nuclear proteins (7 μg) were incubated with a 32P-labeled TBE oligonucleotide for 30 min at room temperature in reaction buffer (20 mM HEPES, pH 7.6, 75 mM KCl, 0.2 mM EDTA, 20% glycerol) and 1 μg of poly(dI-dC)-poly(dI-dC) (Pharmacia) as a nonspecific competitor. Protein-DNA complexes were separated from the free probe on 4% nondenaturing polyacrylamide gel and visualized by autoradiography.
Immunoblot analysis.
Human fetal type II cells were cultured for up to 48 h in control medium, in medium containing Bt2cAMP (1 mM), or in medium containing IL-1α (10 ng/ml) alone or in combination with Bt2cAMP. In some experiments, type II cells were also incubated in the absence or presence of TSA or d-MTA, as described above. The cells were scraped from the dishes and homogenized in ice-cold phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (1 tablet/10 ml) (Roche Laboratories). Proteins (15 μg) from nuclear and cytoplasmic (supernatant) fractions obtained during the isolation of nuclei for EMSA (47) were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose membranes as described previously (38). The membranes were then analyzed for SP-A, TTF-1, p50, p65, and CBP by immunoblotting using specific antisera (antibodies for SP-A [43] and TTF-1 [32] were generated in our laboratory; antibodies for p50, p65, and CBP [Santa Cruz Biotechnology, Inc.] and histone H3, histone H3 acetylated on lysines 9 and 14, and histone H3 dimethylated on lysine 9 [Upstate Biotechnology, Inc.] were obtained commercially) and an enhanced chemiluminescence system (ECL), according to the manufacturer's recommendations (Amersham).
Real-time reverse transcriptase PCR.
Total RNA was isolated from human fetal lung type II cells cultured in the absence or presence of Bt2cAMP (1 mM) or IL-1α for 24 h in an atmosphere of 2% or 20% O2 by the one-step method described previously Chomczynski and Sacchi (12) (TRIzol; Invitrogen). RNA was treated with DNase to remove any contaminating DNA, and 4 μg was reversed transcribed using random primers and Superscript II RNase H reverse transcriptase (Invitrogen). Validated primer sets directed against HDACs 1 to 11, NCoR1, and SMRT along with the constitutively expressed cyclophilin were utilized (Table 1). All primer sets produced amplicons of the expected sizes and sequences. For the quantitative analysis of mRNA expression, the ABI Prism 7700 detection system (Applied Biosystems) was employed with the DNA binding dye SYBR green (PE Applied Biosystems) for the detection of PCR products. The cycling conditions were 50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold was set at a level where the exponential increase in PCR amplification was approximately parallel between all samples. To correct for differences in RNA quantity among samples, data were normalized by using the ratio of the target cDNA concentrations to that of cyclophilin. We have compared expression of cyclophilin to that of 18S rRNA and have found both to be unaffected by treatment or changes in O2 tension.
TABLE 1.
Primers used in quantitative real-time PCR
| Primer name | NCBI accession no. | Forward primer | Reverse primer |
|---|---|---|---|
| HDAC-1 | BC000301 | 5′-GAACCTTAGAATGCTGCCGC-3′ | 5′-CAGGGTCGTCTTCGTCCTCA-3′ |
| HDAC-2 | BC031055 | 5′-GGAGCCCATGGCGTACAGT-3′ | 5′-TTCATGGGATGACCCTGTCC-3′ |
| HDAC-4 | AF132607 | 5′-TACATGTCCCTCCACCGCTAC-3′ | 5′-AGCCATGTTGACGTTGAAACC-3′ |
| HDAC-11 | BC009676 | 5′-TCATGGATGTCTACAACCGCC-3′ | 5′-CTCATCATCCTCTGTGCCCC-3′ |
| Cyclophilin | NM021130 | 5′-TTTCATCTGCACTGCCAAGA-3′ | 5′-TTGCCAAACACCACATGCT-3′ |
| NCoR1 | NM006311 | 5′-TTCGCAGTCCCTGATTATCGT-3′ | 5′-AAGGAAGGTCGCCTTCGAAG-3′ |
| SMRT | NM006312 | 5′-CATGAACGGGCTTATGGCC-3′ | 5′-TGCATGAACTTCTCCCGGA-3′ |
ChIP.
Human fetal type II cells were cultured for up to 48 h in serum-free medium, in medium containing Bt2cAMP (1 mM), or in medium containing IL-1α (10 ng/ml) alone or in combination with Bt2cAMP. Chromatin immunoprecipitation (ChIP) was performed using a modification (11) of previously published methods (29). Briefly, the type II cells (1 × 107 to 1.5 × 107 cells) were washed once with PBS and then incubated with 1% formaldehyde (in control medium) for 10 min at room temperature to cross-link proteins and DNA. Cross-linking was terminated by the addition of glycine (0.125 M final concentration). The cells were then washed twice with cold PBS and placed in 500 μl of “lysis buffer” (25 mM Tris, pH 8.1, 140 mM NaCl, 1% Triton X-100, 0.1% SDS, protease inhibitor cocktail [Roche], and 3 mM EDTA). The lysates from several dishes were combined and sonicated on ice to produce sheared, soluble chromatin. The soluble chromatin was then aliquoted into 500-μl amounts and incubated with antibodies for TTF-1 (31), p65, p50 (Santa Cruz), acetylated histone H3 (lysines 9 and 14; Upstate Biotechnology, Inc.), dimethyl histone H3 (lysine 9), SRC-1, or CBP (Santa Cruz Biotechnology, Inc.) and incubated at 4°C overnight. Two aliquots were reserved as controls: one was incubated without antibody, and the other was incubated with nonimmune immunoglobulin G. Protein A/G Plus agarose beads (60 μl) were then added to each tube, the mixtures were incubated for 2 h at 4°C, and the immune complexes were collected by centrifugation. The beads containing the immunoprecipitated complexes were then washed sequentially for 5 to 10 min in wash buffer I (20 mM Tris-HCl, pH 8.1, 2 mM EDTA, 0.1% SDS, 1% Triton X-100, 150 mM NaCl), wash buffer II (same as wash buffer I except that it contained 500 mM NaCl), wash buffer III (10 mM Tris-HCl, pH 8.1, 1 mM EDTA, 1% NP-40, 1% deoxycholate, 0.25 M LiCl), and 2× Tris-EDTA buffer. The beads were then eluted with 250 μl of elution buffer (1% SDS, 0.1 mM NaHCO3 plus 20 μg salmon sperm DNA [Sigma]) at room temperature. This procedure was repeated once, and eluates were combined. Cross-linking of the immunoprecipitated chromatin complexes and “input controls” (5% of the total soluble chromatin) was reversed by heating the samples at 65°C for 4 h. Proteinase K (15 μg; Invitrogen) was then added to each sample in buffer (50 mM Tris-HCl, pH 8.5, 1% SDS, 10 mM EDTA) and incubated for 1 h at 45°C. The DNA was purified by phenol-chloroform extraction and precipitated in ethyl alcohol for at least 30 min at −20°C. Samples and “input” controls were diluted in 10 to 100 μl Tris-EDTA buffer just prior to PCR. Real-time PCR (14) was carried out using 5′ (5′-TTTTTCTTTACCAGGTTCTGTGCTGCTC-3′) and 3′ (5′-CACATTTCCCTGCAGAACACTA-3′) primers that amplify a 75-bp region of the hSP-A2 5′-flanking region surrounding the TBE.
RESULTS
Cyclic AMP and IL-1 enhance recruitment of TTF-1 and NF-κB p65 to the SP-A gene promoter.
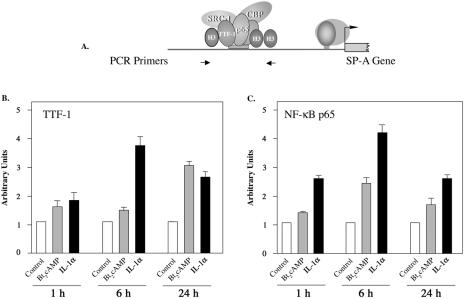
Previously, we observed that Bt2cAMP and IL-1 increased SP-A expression in human fetal type II cells and that these effects were mediated by increased binding of TTF-1 and NF-κB in type II cell nuclear extracts to the TBE (20). In the present study, ChIP was utilized to analyze the time-dependent effects of cAMP and IL-1 on in vivo binding of TTF-1 and NF-κB p65 to this element. Human fetal lung type II cells were cultured in the absence or presence of Bt2cAMP or IL-1α for 1, 6, or 24 h. After immunoprecipitation of chromatin complexes, quantitative real-time PCR was used to amplify a 75-bp genomic region surrounding the TBE within the 5′-flanking region of the SP-A gene (Fig. 1A). As can be seen in Fig. 1B and C, IL-1α stimulated the recruitment of TTF-1 and p65 to the TBE, and this effect was maximal within 6 h. The stimulatory effect of IL-1α on the recruitment of p65 to the TBE was observed as early as 1 h after the start of treatment. Cyclic AMP also increased the recruitment of TTF-1 and p65 to the TBE; however, the cAMP-stimulated recruitment of TTF-1 was first observed at 24 h, while cAMP stimulation of p65 recruitment was evident by 6 h.
FIG. 1.
Cyclic AMP and IL-1α enhance recruitment of TTF-1 and NF-κB p65 to the SP-A gene promoter. Type II cells were cultured for 24 h in control medium in a 2% or 20% O2 environment and then incubated for 1, 6, or 24 h with Bt2cAMP (1 mM) or IL-1α (10 ng/ml) in 2% or 20% O2. The cells were then treated with 1% formaldehyde followed by lysis and sonication to shear and solubilize the cross-linked chromatin, which was immunoprecipitated using antibodies specific for NF-κB p65 and TTF-1. DNA was purified, and the relative abundance of a 75-bp region surrounding the TBE was quantified by real-time PCR using primers indicated by the arrows in panel A. Panel B is representative of four independent experiments with comparable results. The data are expressed as arbitrary units. The bars represent the means ± standard errors of the means (SEM) of values from three sets of culture dishes.
Hypoxia inhibits cAMP and IL-1 induction of SP-A expression and binding of type II cell nuclear proteins to the TBE.
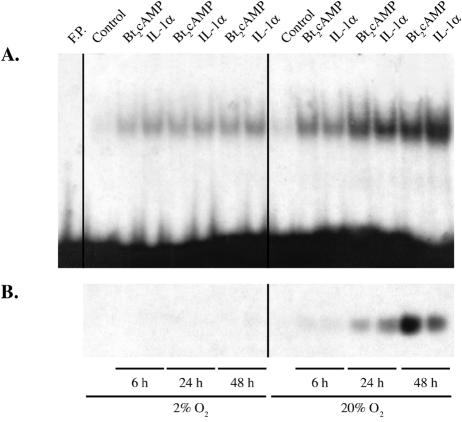
In previous studies, we found that a critical O2 tension was required for type II cell differentiation and cAMP induction of SP-A gene expression in human fetal lung maintained in organ culture; these effects of hypoxia were reversible (1). To determine whether hypoxia also prevented IL-1 stimulation of SP-A expression and to begin to define mechanisms for these effects, human fetal type II cells were incubated for 6, 24, or 48 h in the absence or presence of Bt2cAMP or IL-1α in a 2% or 20% O2-containing environment. In this experiment, all type II cells were incubated for 48 h in the serum-free culture medium; Bt2cAMP and IL-1α were added to the medium at 6, 24, or 48 h prior to harvesting of the cells for analysis. Type II cell nuclear proteins were analyzed for DNA-binding activity by EMSA using the radiolabeled TBE as a probe. Cytoplasmic fractions from the same cells were analyzed for SP-A protein levels by immunoblotting.
As can be seen in the immunoblot in Fig. 2B, when human fetal lung type II cells were cultured in a 20% O2 environment, equivalent inductive effects of Bt2cAMP and IL-1α on SP-A expression were evident at 6 and 24 h. By 48 h, the stimulatory effect of Bt2cAMP was greater than that of IL-1α. On the other hand, when the cells were cultured in 2% O2, effects of Bt2cAMP and IL-1α were barely detected. As shown in the EMSA in Fig. 2A, when type II cells were cultured in a 20% O2 environment, cAMP and IL-1α had pronounced stimulatory effects on the binding of type II cell nuclear extracts to the TBE at all time points. In contrast to the SP-A immunoblot, after 48 h of incubation, IL-1α had a more pronounced stimulatory effect on TBE-binding activity than Bt2cAMP. It should be noted that the effects of cAMP on SP-A promoter activity are due to the cooperative interaction of transcription factors binding to a number of different response elements, including a nuclear receptor response element (2, 39, 56), an E box that binds USF1/2 (18), and a GT box, which binds Sp1 (57). Mutagenesis of any one of these elements abrogates cAMP induction of SP-A gene expression. Thus, whereas the effect of IL-1 may be due primarily to NF-κB binding to the TBE, the effect of cAMP is exerted through a number of different transcription factors and response elements. The stimulatory effects of cAMP and IL-1α on TBE-binding activity were decreased when type II cells were cultured under hypoxic conditions, compared to their effects in cells cultured in 20% O2.
FIG. 2.
Hypoxia inhibits cAMP and IL-1 induction of SP-A expression and binding of type II cell nuclear proteins to the TBE. Human fetal lung type II cells were cultured for 24 h in control medium and then incubated for 6, 24, or 48 h in the absence or presence of Bt2cAMP or IL-1α in either a 2% or 20% O2-containing environment. Nuclear proteins (7 μg) from the type II cells were analyzed for binding activity by EMSA using the radiolabeled TBE as a probe (A). Cytoplasmic fractions (15 μg protein) from the same cells were analyzed for SP-A protein levels by immunoblotting (B). Shown are autoradiograms of a representative immunoblot and gel shift from an experiment that was repeated four times with similar results. F.P., free probe.
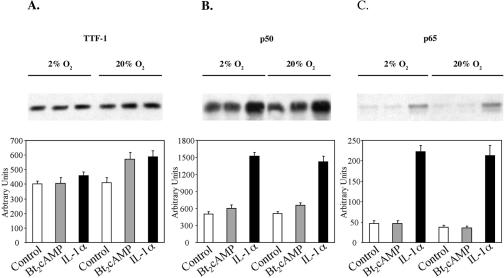
TTF-1 and NF-κB protein levels in fetal lung type II cells are not altered by hypoxia.
To determine whether the effects of O2 tension on cAMP and IL-1 stimulation of TBE-binding activity were correlated with nuclear levels of TTF-1 and NF-κB, we analyzed nuclear levels of TTF-1, p65, and p50 by immunoblotting. Human fetal type II cells were incubated for 6 h in control medium or in medium containing Bt2cAMP or IL-1α in either a 2% or 20% O2-containing environment. As can be seen in Fig. 3 and as observed previously (20), treatment of type II cells with IL-1 caused an increase in nuclear levels of p50 and p65, whereas TTF-1 levels were unaffected; Bt2cAMP had no effect to alter nuclear localization of TTF-1 or NF-κB proteins. Importantly, the actions of IL-1α to increase nuclear levels of p50 and p65 after 6 h of incubation were unaffected by O2 tension (Fig. 3). Collectively, these findings suggest that the inhibitory effects of hypoxia on the binding of type II cell nuclear proteins to the TBE are likely mediated by actions independent of expression or nuclear translocation (in the case of NF-κB) of these transcription factors. Such effects could include alterations in the posttranslational modification of these proteins with associated changes in the recruitment of coactivators.
FIG. 3.
TTF-1, p65, and p50 levels in human fetal lung type II cells are not altered by hypoxia. Human fetal type II cells were incubated for 24 h in control medium in 2% or 20% O2 followed by incubation for 6 h in the absence (control) or presence of Bt2cAMP or IL-1α in either a 2% or 20% O2-containing environment. Nuclear proteins (15 μg) were analyzed by immunoblotting using antisera to TTF-1 (A), p50 (B), and p65 (C). Shown in the upper panel are autoradiograms of immunoblots from a representative experiment. Shown in the lower panel are summarized results of densitometric scans of the immunoblots. Scanned values for the treated samples in each set are plotted relative to those of the control sample in that set. Data are the means ± SEM values from three independent scans.
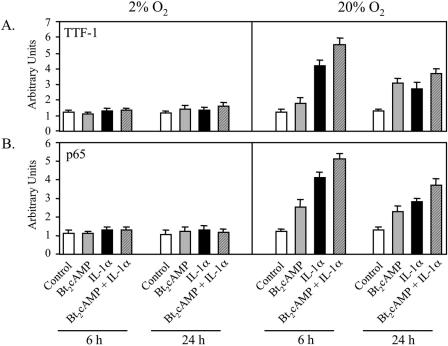
Increased O2 tension is required for cAMP- and IL-1-induced recruitment of TTF-1 and NF-κB to the TBE.
ChIP was used to analyze the effects of O2 tension on cAMP- and IL-1-induced recruitment of TTF-1 and NF-κB p65 to the region of the SP-A promoter surrounding the TBE in vivo. Type II cells were incubated for 24 h in control medium in a 2% or 20% O2 environment and then cultured for 6 or 24 h with cAMP or IL-1α, alone or in combination. As we also observed in the experiment shown in Fig. 1, when type II cells were cultured in a 20% O2 environment, cAMP and IL-1α enhanced recruitment of TTF-1 and p65 to the TBE. By contrast, when cells were cultured under hypoxic conditions, cAMP and IL-1 stimulation of in vivo binding activity was prevented (Fig. 4A and B). A similar effect of O2 tension was observed for the recruitment of p50 to the TBE (data not shown). These findings indicate that a critical O2 tension is essential for cAMP and IL-1 stimulation of binding of TTF-1 and NF-κB to the SP-A promoter.
FIG. 4.
Effects of cAMP and IL-1 to increase recruitment of TTF-1 and NF-κB to the TBE are inhibited by hypoxia. Type II cells were incubated for 24 h in control medium in a 2% or 20% O2 environment and then treated for another 6 or 24 h with Bt2cAMP or IL-1α, alone or in combination. After cross-linking, lysis, and sonication, the solubilized chromatin was subjected to immunoprecipitation using specific antibodies to NF-κB and TTF-1. After immunoprecipitation, DNA was purified and analyzed for the 75-bp region containing the TBE (Fig. 1A) using real-time PCR. The data are expressed as arbitrary units. Shown is a representative of four independent experiments. The bars represent the means ± SEM of values from three sets of culture dishes.
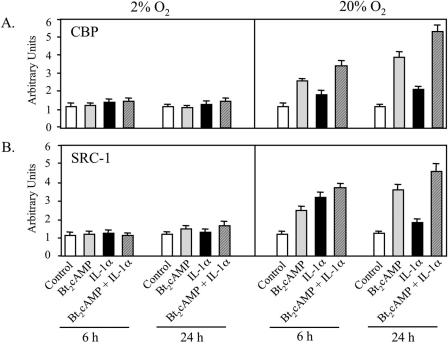
Increased O2 tension is required for cAMP- and IL-1-induced recruitment of CBP and SRC-1 to the TBE.
Previously, we observed that TTF-1 synergistically interacted with NF-κB (20) and with the coactivators CBP and SRC-1 (55) at the TBE to increase SP-A promoter activity. TTF-1 also was found to physically interact with NF-κB in vivo (20) and with these coactivators in vitro (55). CBP and SRC-1 have also been reported to interact with p65 (19) and p50 (40), respectively. In the present study, ChIP was used to define the role of O2 tension on in vivo binding of CBP and SRC-1 to the region of the SP-A promoter containing the TBE. Human fetal lung type II cells were cultured in control medium or in medium containing Bt2cAMP or IL-1α for 6 or 24 h at 2% and 20% O2. As can be seen in Fig. 5A and B, when the type II cells were cultured in 20% O2, cAMP and IL-1α enhanced the recruitment of CBP and SRC-1 to the TBE. A greater stimulatory effect of cAMP plus IL-1 was also evident at both time points. Differential effects of cAMP and IL-1 on the recruitment of CBP and SRC-1 were observed at the 6- and 24-h time points; this may be due, in part, to differences in the kinetics of cycling of transcription factors and coactivators on and off the DNA. Notably, when the cells were cultured in a 2% O2 environment, the stimulatory effects of cAMP on CBP and SRC-1 recruitment were greatly reduced.
FIG. 5.
Cyclic AMP- and IL-1-induced recruitment of CBP and SRC-1 to the TBE is inhibited by hypoxia. Type II cells were incubated for 24 h in control medium in a 2% or 20% O2-containing environment and then incubated for another 6 or 24 h with cAMP or IL-1, alone or in combination. The soluble cross-linked chromatin was immunoprecipitated using specific antibodies to CBP and SRC-1. After immunoprecipitation, DNA was analyzed for the 75-bp region containing the TBE (Fig. 1A) using real-time PCR. The data are expressed as arbitrary units. Shown is a representative of four independent experiments. The bars represent the means ± SEM of values from triplicate dishes of type II cells.
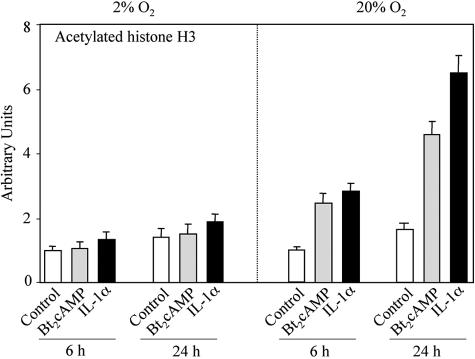
Increased O2 tension facilitates cAMP induction of histone H3 acetylation at the TBE.
In light of the fact that both CBP and SRC-1 have histone acetylase activity, the effects of O2 tension on the acetylation of histones at the SP-A promoter in the region of the TBE were analyzed. ChIP assays were carried out using antibodies to histone H3 acetylated on lysines 9 and 14. Human fetal lung type II cells were cultured in the absence or presence of Bt2cAMP or IL-1α for 6 or 24 h in a 2% or 20% O2 environment. When type II cells were cultured in a 20% oxygen environment, cAMP and IL-1α treatment caused a marked increase in local acetylation of histone H3 after 6 and 24 h of incubation (Fig. 6). By contrast, when the cells were cultured in 2% O2, no stimulatory effects of cAMP or IL-1α were evident (Fig. 6). On the other hand, basal levels of acetylated histone H3 were essentially unaffected by changes in O2 tension. Taken together, these findings suggest that O2 serves a permissive role in cAMP- and IL-1-stimulated recruitment of coactivators with histone acetylase activity to the SP-A promoter. The cAMP-induced recruitment of coactivators likely mediates increased local histone acetylation and an opening of the chromatin structure leading to increased SP-A gene transcription.
FIG. 6.
O2 is required for cAMP induction of histone H3 acetylation at the TBE. Human fetal lung type II cells were cultured in control medium for 24 h in a 2% or 20% O2-containing environment followed by incubation in the absence (control) or presence of Bt2cAMP or IL-1α for 6 or 24 h in 2% or 20% O2. The solubilized chromatin was subjected to immunoprecipitation using an antibody to acetylated histone H3. After immunoprecipitation, DNA was purified and analyzed for the 75-bp region containing the TBE (Fig. 1A) by real-time PCR. Data are expressed as arbitrary units. Shown is a representative of three independent experiments. The bars represent the means ± SEM of data from triplicate culture dishes.
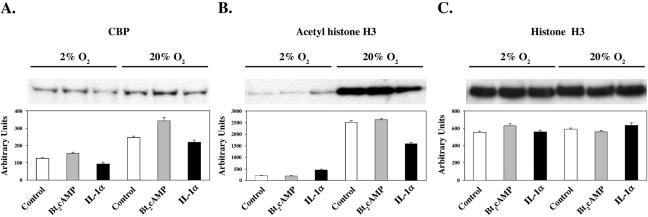
Increased O2 tension enhances nuclear levels of CBP and of acetylated histone H3 in human fetal lung type II cells.
In consideration of the permissive effects of O2 on cAMP-induced recruitment of CBP, SRC-1, and acetylated histone H3 to the genomic region containing the TBE in cAMP-treated type II cells, nuclear levels of immunoreactive CBP and of acetylated and total histone H3 were analyzed in type II cells cultured in the absence or presence of Bt2cAMP or IL-1α in 2% or 20% O2. As can be seen in Fig. 7, nuclear levels of immunoreactive CBP and acetyl-histone H3 were increased in cells cultured in 20% O2 compared to levels in cells cultured in 2% O2. The stimulatory effects of O2 were independent of treatment. By contrast, levels of total histone H3 were unaffected by O2 tension. This suggests that permissive effects of increased O2 tension on cAMP and cytokine stimulation of SP-A gene expression are mediated, in part, by global increases in histone acetylase activity that are independent of hormonal treatment as well as hormone-dependent effects on the binding of TTF-1, NF-κB, and coactivators to the SP-A promoter with local enhancement of histone acetylation.
FIG. 7.
Levels of immunoreactive CBP and acetylated histone H3 in human fetal type II cells are reduced by hypoxia. Human fetal type II cells were incubated for 24 h in control medium in a 2% or 20% O2-containing environment followed by incubation for 24 h in the absence or presence of Bt2cAMP or IL-1α in 2% or 20% O2. Nuclear proteins (15 μg) were analyzed by Western blotting using antibodies to CBP (A), acetyl-histone H3 (B), and total histone H3 (C). Shown in the upper panel are autoradiograms of immunoblots from representative experiments. Shown in the lower panel are densitometric scans of the immunoblots. Scanned values for the treated samples in each set are plotted relative to those of the control sample in that set. Data are the means ± SEM from three independent scans.
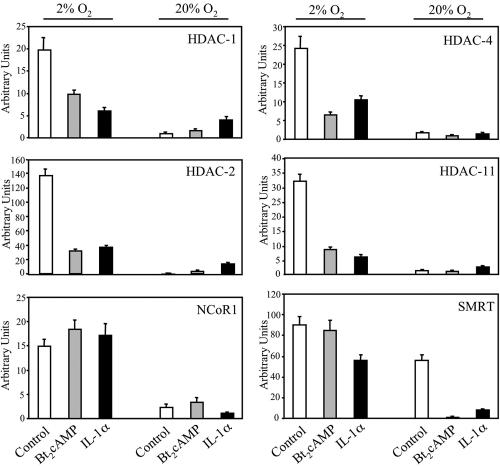
Hypoxia increases corepressor mRNA levels in cultured type II cells.
Real-time reverse transcriptase PCR was used to analyze mRNA levels for HDACs 1 to 11, NCoR1, and SMRT in human fetal type II cells cultured for 24 h in control medium followed by incubation for 24 h in the absence (control) or presence of Bt2cAMP or IL-1α in 2% or 20% O2. Remarkably, the effects of O2 tension and hormonal treatment were essentially the same for all corepressors analyzed. Findings for HDACs 1 and 2 (representative of class I), HDAC-4 (representative of class II), HDAC-11 (representative of class IV), NCoR1, and SMRT are shown in Fig. 8. mRNA levels of these corepressors were markedly induced by hypoxia in type II cells cultured in control medium. Interestingly, cAMP and IL-1α partially antagonized the inductive effects of hypoxia on HDAC expression. These findings suggest that hypoxia promotes a closed chromatin structure through global induction of histone deacetylation.
FIG. 8.
Hypoxia increases corepressor mRNA levels. Human fetal lung type II cells were cultured in control medium for 24 h in a 2% or 20% O2-containing environment followed by incubation in the absence (control) or presence of Bt2cAMP or IL-1α for 24 h in 2% or 20% O2. Total RNA prepared from these cells was analyzed for levels of HDACs 1 to 11 and for NCoR1 and SMRT by quantitative PCR using SYBR green (PE Applied Biosystems) for detection of PCR products. Shown are data for HDACs 1 and 2 (representative of class I), HDAC-4 (representative of class II), HDAC 11 (representative of class IV), NCoR1, and SMRT. Data were normalized by analyzing the ratio of the target cDNA concentrations to that of cyclophilin. Shown is a representative of three independent experiments. The bars represent the means ± SEM of data from triplicate culture dishes.
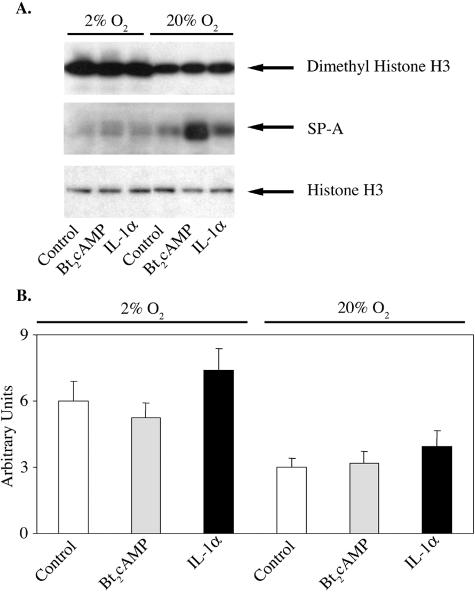
Hypoxia increases nuclear levels of histone H3 dimethylated on Lys9 and enhances levels of dimethyl (Lys9) histone H3 associated with the TBE region of the SP-A promoter.
Human fetal type II cells were incubated for 24 h in control medium followed by incubation for 24 h in the absence (control) or presence of Bt2cAMP or IL-1α in either a 2% or 20% O2-containing environment. Nuclear and cytoplasmic fractions were analyzed for histone H3 dimethylated on Lys9 and for SP-A, respectively, by immunoblotting. As can be seen in Fig. 9, nuclear levels of dimethyl (Lys9) histone H3 were increased in cells incubated in 2% O2 compared to levels in cells incubated in 20% O2 for all treatment groups (Fig. 9A, upper panel), while levels of total histone H3 were unaffected (Fig. 9A, lower panel). Effects of hypoxia on levels of dimethylated H3 were correlated with the inhibitory effects of hypoxia on cAMP and IL-1α induction of SP-A expression (Fig. 9A, middle panel). The hypoxia-induced increase in total nuclear levels of dimethyl (Lys9) H3 was associated with increased levels of dimethyl (Lys9) H3 associated with the 75-bp genomic region surrounding the hSP-A TBE, as revealed by ChIP (Fig. 9B).
FIG. 9.
Nuclear levels of dimethyl histone H3 (K9) and association of dimethyl H3 (K9) with the TBE-containing region of the SP-A promoter are increased by hypoxia. Human fetal lung type II cells were cultured in control medium for 24 h in a 2% or 20% O2-containing environment followed by incubation in the absence (control) or presence of Bt2cAMP or IL-1α for 24 h in 2% or 20% O2. (A) Nuclear and cytoplasmic proteins were analyzed by immunoblotting using antisera to dimethyl histone H3 (K9) (upper panel), SP-A (middle panel), and total histone H3 (lower panel). (B) Solubilized chromatin isolated from the cells was subjected to immunoprecipitation using an antibody to dimethyl histone H3 (K9). After immunoprecipitation, DNA was purified and analyzed by real-time PCR for the 75-bp region containing the TBE. The data are expressed as arbitrary units. Shown is a representative of three independent experiments. The bars represent the means ± SEM of data from triplicate culture dishes.
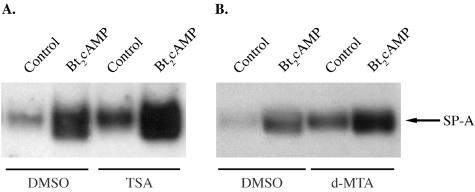
The histone deacetylase inhibitor TSA and the protein methylation inhibitor d-MTA increase SP-A expression in cultured type II cells.
Human fetal lung type II cells were cultured for 24 h in 20% O2 in the presence of DMSO vehicle or with TSA (50 nM) or d-MTA (300 μM) dissolved in DMSO followed by coincubation with or without (control) Bt2cAMP for an additional 24 h. Cytoplasmic fractions were analyzed for SP-A by immunoblotting. As can be seen, both TSA and d-MTA dramatically increased the levels of SP-A in the absence or presence of Bt2cAMP compared to levels in cells incubated with DMSO alone (Fig. 10A and B). These findings suggest that SP-A expression is enhanced by increased acetylation and decreased methylation of histone H3 (Lys9).
FIG. 10.
The histone deacetylase inhibitor TSA and the protein methylation inhibitor d-MTA increase SP-A expression in lung type II cells. Human fetal lung type II cells were cultured in medium in the presence of DMSO vehicle or with TSA (50 nM) or d-MTA (300 μM) dissolved in DMSO for 24 h in a 20% O2 environment followed by coincubation in the absence (control) or presence of Bt2cAMP for an additional 24 h. Cytoplasmic fractions were analyzed for SP-A by immunoblotting. Shown is a representative immunoblot of this experiment, which was repeated three times with comparable results.
DISCUSSION
The activating signals and molecular mechanisms that mediate temporal and spatial regulation of SP-A gene expression in the fetal lung remain to be elucidated. We previously observed that cAMP and IL-1 stimulation of SP-A gene expression in human fetal lung is mediated via TTF-1 and NF-κB binding to a response element (TBE) 175 bp upstream of the transcription initiation site of the hSP-A gene (20, 31). Since TTF-1 is expressed from the very earliest stages of lung development (30), where it plays a role in branching morphogenesis (26), while SP-A gene expression is induced only after ∼80% of gestation is complete (37), we suggest that temporally distinct, gene-specific actions of TTF-1 could be mediated by selective changes in its posttranslational modification and association with other transcriptional regulators. Our finding that cAMP stimulation of type II cell differentiation and SP-A gene expression is dependent upon an O2 tension of ≥10% (1) has led us to consider the possibility that increased O2 availability to the alveolar epithelium as a result of vascularization of the developing lung may play a permissive role in the activation of TTF-1 and NF-κB.
Transcriptional activation of TTF-1 and NF-κB proteins is known to be regulated by phosphorylation. Cyclic AMP stimulation of type II cell nuclear protein binding to the TBE was found to be associated with increased TTF-1 phosphorylation; moreover, phosphatase treatment of type II cell nuclear extracts abolished DNA-binding activity (31). Furthermore, the PKA catalytic subunit increased TTF-1 transcriptional activation of the SP-A (31) and SP-B (54) promoters. Cytokines (42) and PKA (58, 59) have also been reported to increase phosphorylation and DNA-binding and transcriptional activities of NF-κB p65. Phosphorylation of p65 by PKA increased its transcriptional activity by enhancing p65 association with CBP (59). CBP and SRC-1 also act synergistically with TTF-1 to increase SP-A promoter activity, and this was enhanced by cotransfection of the PKA catalytic subunit (55). Our finding that cAMP treatment of type II cells caused a marked increase in TTF-1 acetylation (55) suggests that PKA-mediated phosphorylation of TTF-1 enhances its interaction with coactivators with HAT activity in vivo. The resulting increase in TTF-1 acetylation may further increase DNA-binding and transcriptional activity (21). In light of these collective findings, we postulate that phosphorylation of TTF-1 and NF-κB proteins in response to activating hormones and factors may facilitate their TBE binding and recruitment of the histone acetylases CBP and SRC-1.
By use of ChIP, we observed in the present study that cAMP and IL-1 stimulation of SP-A gene expression in human fetal lung type II cells was associated with enhanced in vivo recruitment of TTF-1 and NF-κB p65 to the genomic region containing the TBE. IL-1 had a rapid (as early as 1 h) effect to promote increased binding of p65 and TTF-1 to the TBE, reaching maximal levels by 6 h. Cyclic AMP also stimulated in vivo binding of p65 and TTF-1 to the TBE-containing region, but this was evident after a longer latency period. These findings agree with previously published in vitro studies using EMSA in which IL-1 caused a marked increase in the binding of type II cell nuclear proteins to the TBE within 1 h of treatment, whereas a stimulatory effect of cAMP on TBE-binding activity was first detectable after 6 h (20).
Cyclic AMP and IL-1 stimulation of SP-A expression and in vitro binding of nuclear proteins to the TBE were inhibited when the cells were cultured in a hypoxic (2% O2) environment. Using ChIP, we also observed that when the type II cells were cultured in 2% O2, cAMP and IL-1 stimulation of in vivo recruitment of TTF-1 and p65 to the TBE was prevented. These findings support the concept that ROS serve a permissive role in transcriptional activation of NF-κB in response to cytokine signaling (33) and suggest that ROS are also permissive for TTF-1 transcriptional activation. Our previous observation that the antioxidant pyrrolidine dithiocarbamate, a known NF-κB inhibitor, blocked IL-1 and cAMP induction of type II cell nuclear protein binding to the TBE and reduced SP-A expression (20) further indicates a requirement for ROS in NF-κB and/or TTF-1 activation of SP-A gene transcription. As mentioned above, phosphorylation appears to be required for the binding of type II cell nuclear proteins to the TBE (31). Thus, the finding that cAMP and IL-1 stimulation of TTF-1 and NF-κB binding to the TBE region was inhibited when type II cells were cultured in 2% O2 compared to 20% O2 suggests that phosphorylation of these proteins may be reduced by hypoxia. This could be due a decreased rate of phosphorylation and/or an increased rate of dephosphorylation of these proteins. It should be noted that hypoxia has been reported to increase activities of the protein phosphatases protein phosphatase 1 (28), calcineurin (34), and tyrosine phosphatase (16) in a number of cell types.
TTF-1 (55), NF-κB p50 (40), and NF-κB p65 (19, 59) were previously found to interact with the coactivators CBP and SRC-1 in vitro. In the present study, we observed using ChIP that cAMP and IL-1 stimulated in vivo recruitment of CBP and SRC-1 to the SP-A genomic region containing the TBE in type II cells cultured in 20% O2; this was prevented when type II cells were cultured in a hypoxic (2% O2) environment. The O2-dependent increase in the recruitment of CBP to the genomic region surrounding the TBE was also associated with global effects on overall nuclear levels of CBP protein. Immunoreactive levels of CBP were elevated in nuclei of type II cells cultured in a 20% O2 environment compared to those in cells cultured in a 2% O2 environment; however, in contrast to our findings using ChIP, this inductive effect of O2 was independent of hormonal treatment. On the other hand, mRNA levels for HDACs 1 to 11, NCoR1, and SMRT were markedly induced by hypoxia in type II cells incubated in control medium. Treatment with Bt2cAMP and IL-1α partially antagonized the inductive effect of hypoxia on HDAC mRNA expression, whereas these factors had little or no effect on HDAC mRNA levels in cells cultured in 20% O2. In this regard, oxidative stress has been reported to increase acetylation of core histones (44) through the inhibition of histone deacetylases (22).
Both CBP and SRC-1 coactivators catalyze the acetylation of specific lysine residues in the N-terminal tails of the nucleosomal core histones (35), resulting in their decreased affinity for DNA and local unwinding of DNA around the nucleosome. This opening of the chromatin structure facilitates recruitment to the promoter of transcription factors and of RNA polymerase II, resulting in the stabilization of the preinitiation complex and activation of transcription initiation (35). Corepressors, on the other hand, inhibit transcriptional activation by mediating deacetylation of histones to promote the formation of a closed chromatin structure. Histones are subject to a number of other posttranslational modifications, including methylation, phosphorylation, ubiquitylation, and ADP-ribosylation (see reference 23 for a review). It has been suggested that the combinatorial nature of these covalent modifications of the histone tails reveals a “histone code,” which provides a unique regulatory system that dictates the transition between transcriptionally silent (heterochromatin) and active (euchromatin) chromatin (23). Whereas euchromatin is generally associated with histones acetylated on specific lysine residues (e.g., lysine 9 of histone H3 [H3-K9] and H3-K14), heterochromatin contains predominately hypoacetylated histones.
In the present study, using ChIP, we observed in cultured type II cells that the cAMP- and IL-1-induced binding of TTF-1 and p65 as well as of CBP and SRC-1 coactivators to the SP-A promoter was associated with an O2-dependent increase in the in vivo binding of H3-K9, a marker of active chromatin (23). By contrast, in cells cultured under hypoxic conditions, cAMP and IL-1α had no effect to enhance recruitment. Global levels of acetylated H3-K9 were also markedly reduced in type II cells cultured in 2% O2. This inhibitory effect of hypoxia on H3-K9 acetylation was associated with increased expression of HDACs 1 to 11 as well as NCoR1 and SMRT mRNAs. Importantly, incubation of type II cells with the HDAC inhibitor TSA enhanced SP-A expression in type II cells cultured in the absence or presence of Bt2cAMP. Based on these findings, we postulate that permissive effects of increased O2 tension on cAMP and cytokine stimulation of SP-A gene expression are mediated, in part, by global inhibition of HDAC expression. In this “permissive” environment, cAMP- and IL-1-induced phosphorylation of TTF-1 and NF-κB results in their increased binding to the TBE, facilitating the recruitment of histone acetylases such as CBP and SRC-1. This in turn causes acetylation of core histones (e.g., H3-K9) in the TBE-containing region of the SP-A promoter, resulting in a further opening of the chromatin structure.
Methylation of specific lysine residues in histones (e.g., H3-K9) has been found to play a role in transcriptional silencing (23) by forming a binding site for heterochromatin protein 1; this subsequently mediates the formation of the condensed structure of heterochromatin (15, 27). In Schizosaccharomyces pombe, methylation of H3-K9 occurs after K9 is deacetylated by the action of an HDAC complex (41). On the other hand, methylation of H3-K4 (23) and methylation of arginine residues (49) have been implicated in transcriptional activation. In the present study, we found that hypoxia increased the recruitment of histone H3 dimethylated on lysine 9 to the SP-A TBE-containing region. Hypoxia also caused a global increase in dimethylated H3-K9. Notably, d-MTA, an S-adenosylmethionine metabolite that inhibits histone H3 methylation (reference 48 and data not shown), markedly increased SP-A expression in type II cells cultured in the absence or presence of Bt2cAMP. This further supports the inhibitory role of histone H3 (Lys9) methylation in SP-A gene expression. Unlike acetylation, methylation does not remove the positive charge of the lysine but increases bulkiness by the addition of one, two, or three methyl groups to its ɛ-amino group. The significance of the number of methyl groups is not understood, but it has been suggested to add a level of complexity to the histone code (23).
Although histone acetylation, phosphorylation, and ubiquitylation are dynamic and reversible, methylation has long been thought to be essentially irreversible and involved in long-term maintenance of a repressed transcriptional state (23). Recently, it has been found that methylation of certain histone residues (e.g., H3-K9) in a number of inflammatory response genes is also subject to rapid changes (45). Interestingly, a lysine-specific histone demethylase, Epe1, that alters heterochromatin integrity in yeast has been described (6). Epe1 is structurally related to the 2-OG-Fe(II)-dependent dioxygenase superfamily and has been proposed to catalyze hydroxylation of the methyl groups on histone tails, resulting in oxidative demethylation (51). These findings are of interest in light of the proposed role of O2 and ROS as modulators of cell differentiation (5, 46). It has been postulated that increased vascularization of tissues with associated enhanced O2 delivery plays a role in cellular differentiation with the associated loss of mitotic activity (1, 10, 24). Cellular differentiation in a number of organisms has been found to be associated with an increase in O2 free radicals (5), an induction of the mangano isoform of superoxide dismutase, and a reduction in cellular levels of reduced glutathione (5). In the developing fetal rat lung, a marked decline in levels of glutathione (52) and induction of the mangano isoform of superoxide dismutase activity (50) were observed, suggestive of an increase in tissue O2 tension.
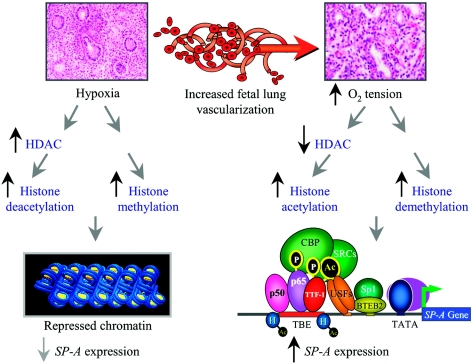
Based on our findings, we suggest that the hypoxic environment that exists within the fetal lung during the first and second trimesters of gestation promotes increased HDAC activity, hypoacetylation, and increased methylation of core histones, resulting in a closed chromatin structure (Fig. 11). During that latter third of gestation, the increased vascularization of the fetal lung and enhanced O2 availability to the pulmonary epithelium may promote histone demethylation and increased histone acetylation, resulting in a “permissive” chromatin structure. The increased O2 tension also facilitates cAMP and IL-1 induction of NF-κB and TTF-1 transcriptional activation and binding to the SP-A promoter. These activated transcription factors in turn recruit CBP and SRC coactivators, which catalyze the acetylation of core histones in the genomic region surrounding the TBE and a further opening of the chromatin structure. This would facilitate increased access of basal transcription factors and polymerase to the SP-A promoter, leading to the developmental activation of gene transcription (Fig. 11). We propose that the present findings may provide a paradigm for transcriptional regulation of other genes that are developmentally regulated in association with increased tissue vascularization and O2 availability.
FIG. 11.
Proposed mechanisms for the developmental induction of SP-A gene transcription in the fetal lung. During the first two-thirds of gestation, the fetal lung is poorly vascularized and relatively hypoxic. Based on the findings of this study, we propose that under hypoxic conditions, there is increased expression of HDACs and enhanced methylation of histones on residues known to mediate a repressed chromatin state (e.g., K9 of histone H3). The SP-A gene is therefore rendered inaccessible to activating transcription factors. During the latter third of gestation, capillary networks form within the fetal lung, resulting in increased O2 availability to the developing pulmonary epithelium. The elevated O2 tension facilitates the inhibition of HDAC expression and the increased acetylation of histones on lysine residues known to mediate an active chromatin state (e.g., K9 of histone H3). This may be further enhanced by demethylation of core histones and DNA. Increased O2 tension also promotes increased phosphorylation, activation, and binding of TTF-1 and NF-κB to the TBE with subsequent recruitment of CBP and SRC coactivators containing intrinsic histone acetylase activity. This results in a local increase in histone acetylation (e.g., K9 of histone H3), resulting in a further opening of the chromatin structure, the formation of a stable basal transcription complex at the promoter with recruitment of RNA polymerase II, and enhanced SP-A gene transcription.
Acknowledgments
This research was supported in part by NIH grants 2-U01-HL-052647 and 5-R37-HL-050022. K.N.I. was supported by NIH grant 5-T32-HD-07190.
We are grateful to Meg Smith for her expert assistance in the preparation of primary cultures of fetal lung type II cells.
REFERENCES
- 1.Acarregui, M. J., J. M. Snyder, and C. R. Mendelson. 1993. Oxygen modulates the differentiation of human fetal lung in vitro and its responsiveness to cAMP. Am. J. Physiol. 264:L465-L474. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn, J. L., E. Gao, Q. Chen, M. E. Smith, R. D. Gerard, and C. R. Mendelson. 1993. Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol. Endocrinol. 7:1072-1085. [DOI] [PubMed] [Google Scholar]
- 3.Alcorn, J. L., R. E. Hammer, K. R. Graves, M. E. Smith, S. D. Maika, L. F. Michael, E. Gao, Y. Wang, and C. R. Mendelson. 1999. Analysis of genomic regions involved in regulation of the rabbit surfactant protein A gene in transgenic mice. Am. J. Physiol. 277:L349-L361. [DOI] [PubMed] [Google Scholar]
- 4.Alcorn, J. L., M. E. Smith, J. F. Smith, L. R. Margraf, and C. R. Mendelson. 1997. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am. J. Respir. Cell Mol. Biol. 17:672-682. [DOI] [PubMed] [Google Scholar]
- 5.Allen, R. G. 1991. Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc. Soc. Exp. Biol. Med. 196:117-129. [DOI] [PubMed] [Google Scholar]
- 6.Ayoub, N., K. Noma, S. Isaac, T. Kahan, S. I. Grewal, and A. Cohen. 2003. A novel jmjC domain protein modulates heterochromatization in fission yeast. Mol. Cell. Biol. 23:4356-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin, A. S. J. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 8.Bohinski, R. J., R. Di Lauro, and J. A. Whitsett. 1994. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 14:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno, M. D., R. J. Bohinski, K. M. Huelsman, J. A. Whitsett, and T. R. Korfhagen. 1995. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J. Biol. Chem. 270:6531-6536. [DOI] [PubMed] [Google Scholar]
- 10.Caplan, A. I., and S. Koutroupas. 1973. The control of muscle and cartilage development in the chick limb: the role of differential vascularization. J. Embryol. Exp. Morphol. 29:571-583. [PubMed] [Google Scholar]
- 11.Chakrabarti, S. K., J. C. James, and R. G. Mirmira. 2002. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J. Biol. Chem. 277:13286-13293. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 13.Condon, J. C., P. Jeyasuria, J. M. Faust, and C. R. Mendelson. 2004. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc. Natl. Acad. Sci. USA 101:4978-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condon, J. C., P. Jeyasuria, J. M. Faust, J. M. Wilson, and C. R. Mendelson. 2003. A decline in progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of labor. Proc. Natl. Acad. Sci. USA 100:9518-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elgin, S. C., and S. I. Grewal. 2003. Heterochromatin: silence is golden. Curr. Biol. 13:R895-R898. [DOI] [PubMed] [Google Scholar]
- 16.Fan, C., Q. Li, D. Ross, and J. F. Engelhardt. 2003. Tyrosine phosphorylation of IκBα activates NF-κB through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J. Biol. Chem. 278:2072-2080. [DOI] [PubMed] [Google Scholar]
- 17.Gao, E., J. L. Alcorn, and C. R. Mendelson. 1993. Identification of enhancers in the 5′-flanking region of the rabbit surfactant protein A (SP-A) gene and characterization of their binding proteins. J. Biol. Chem. 268:19697-19709. [PubMed] [Google Scholar]
- 18.Gao, E., Y. Wang, J. L. Alcorn, and C. R. Mendelson. 2003. Transcription factor USF2 is developmentally regulated in fetal lung and acts together with USF1 to induce SP-A gene expression. Am. J. Physiol. 284:L1027-L1036. [Google Scholar]
- 19.Gerritsen, M. E., A. J. Williams, A. S. Neish, S. Moore, Y. Shi, and T. Collins. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 94:2927-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam, K. N., and C. R. Mendelson. 2002. Potential role of nuclear factor κB and reactive oxygen species in cAMP and cytokine regulation of surfactant protein-A gene expression in lung type II cells. Mol. Endocrinol. 16:1428-1440. [DOI] [PubMed] [Google Scholar]
- 21.Ito, A., C. H. Lai, X. Zhao, S. Saito, M. H. Hamilton, E. Appella, and T. P. Yao. 2001. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20:1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, K., T. Hanazawa, K. Tomita, P. J. Barnes, and I. M. Adcock. 2004. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315:240-245. [DOI] [PubMed] [Google Scholar]
- 23.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B., A. Kamat, and C. R. Mendelson. 2000. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol. Endocrinol. 14:1661-1673. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, S. E., C. J. Bachurski, M. S. Burhans, and S. W. Glasser. 1996. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J. Biol. Chem. 271:6881-6888. [DOI] [PubMed] [Google Scholar]
- 26.Kimura, S., Y. Hara, T. Pineau, P. Fernandez-Salguero, C. H. Fox, J. M. Ward, and F. J. Gonzalez. 1996. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10:60-69. [DOI] [PubMed] [Google Scholar]
- 27.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 28.Krtolica, A., N. A. Krucher, and J. W. Ludlow. 1998. Hypoxia-induced pRB hypophosphorylation results from downregulation of CDK and upregulation of PP1 activities. Oncogene 17:2295-2304. [DOI] [PubMed] [Google Scholar]
- 29.Kuo, M. H., and C. D. Allis. 1999. In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods 19:425-433. [DOI] [PubMed] [Google Scholar]
- 30.Lazzaro, D., M. Price, M. De Felice, and R. Di Lauro. 1991. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113:1093-1104. [DOI] [PubMed] [Google Scholar]
- 31.Li, J., E. Gao, and C. R. Mendelson. 1998. Cyclic AMP-responsive expression of the surfactant protein-A gene is mediated by increased DNA binding and transcriptional activity of thyroid transcription factor-1. J. Biol. Chem. 273:4592-4600. [DOI] [PubMed] [Google Scholar]
- 32.Li, J., E. Gao, S. R. Seidner, and C. R. Mendelson. 1998. Differential regulation of baboon SP-A1 and SP-A2 genes: structural and functional analysis of 5′-flanking DNA. Am. J. Physiol. 275:L1078-L1088. [DOI] [PubMed] [Google Scholar]
- 33.Li, N., and M. Karin. 1999. Is NF-κB the sensor of oxidative stress? FASEB J. 13:1137-1143. [PubMed] [Google Scholar]
- 34.Li, W. E., and J. I. Nagy. 2000. Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. Eur. J. Neurosci. 12:2644-2650. [DOI] [PubMed] [Google Scholar]
- 35.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 36.Mendelson, C. R. 2000. Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu. Rev. Physiol. 62:875-915. [DOI] [PubMed] [Google Scholar]
- 37.Mendelson, C. R., C. Chen, V. Boggaram, C. Zacharias, and J. M. Snyder. 1986. Regulation of the synthesis of the major surfactant apoprotein in fetal rabbit lung tissue. J. Biol. Chem. 261:9938-9943. [PubMed] [Google Scholar]
- 38.Mendelson, C. R., J. M. Johnston, P. C. MacDonald, and J. M. Snyder. 1981. Multihormonal regulation of surfactant synthesis by human fetal lung in vitro. J. Clin. Endocrinol. Metab. 53:307-317. [DOI] [PubMed] [Google Scholar]
- 39.Michael, L. F., J. L. Alcorn, E. Gao, and C. R. Mendelson. 1996. Characterization of the cyclic adenosine 3′,5′-monophosphate response element of the rabbit surfactant protein-A gene: evidence for transactivators distinct from CREB/ATF family members. Mol. Endocrinol. 10:159-170. [DOI] [PubMed] [Google Scholar]
- 40.Na, S. Y., S. K. Lee, S. J. Han, H. S. Choi, S. Y. Im, and J. W. Lee. 1998. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor κB-mediated transactivations. J. Biol. Chem. 273:10831-10834. [DOI] [PubMed] [Google Scholar]
- 41.Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. Grewal. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110-113. [DOI] [PubMed] [Google Scholar]
- 42.Naumann, M., and C. Scheidereit. 1994. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 13:4597-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odom, M. J., J. M. Snyder, and C. R. Mendelson. 1987. Adenosine 3′,5′-monophosphate analogs and β-adrenergic agonists induce the synthesis of the major surfactant apoprotein in human fetal lung in vitro. Endocrinology 121:1155-1163. [DOI] [PubMed] [Google Scholar]
- 44.Rahman, I. 2002. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem. Pharmacol. 64:935-942. [DOI] [PubMed] [Google Scholar]
- 45.Saccani, S., and G. Natoli. 2002. Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev. 16:2219-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauer, H., M. Wartenberg, and J. Hescheler. 2001. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 11:173-186. [DOI] [PubMed] [Google Scholar]
- 47.Snyder, J. M., J. M. Johnston, and C. R. Mendelson. 1981. Differentiation of type II cells of human fetal lung in vitro. Cell Tissue Res. 220:17-25. [DOI] [PubMed] [Google Scholar]
- 48.Song, M. R., and A. Ghosh. 2004. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat. Neurosci. 7:229-235. [DOI] [PubMed] [Google Scholar]
- 49.Stallcup, M. R. 2001. Role of protein methylation in chromatin remodeling and transcriptional regulation. Oncogene 20:3014-3020. [DOI] [PubMed] [Google Scholar]
- 50.Tanswell, A. K., and B. A. Freeman. 1984. Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr. Res. 18:584-587. [DOI] [PubMed] [Google Scholar]
- 51.Trewick, S. C., P. J. McLaughlin, and R. C. Allshire. 2005. Methylation: lost in hydroxylation? EMBO Rep. 6:315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warshaw, J. B., C. W. Wilson, K. Saito, and R. A. Prough. 1985. The responses of glutathione and antioxidant enzymes to hyperoxia in developing lung. Pediatr. Res. 19:819-823. [DOI] [PubMed] [Google Scholar]
- 53.Wright, J. R. 2005. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 5:58-68. [DOI] [PubMed] [Google Scholar]
- 54.Yan, C., and J. A. Whitsett. 1997. Protein kinase A activation of the surfactant protein B gene is mediated by phosphorylation of thyroid transcription factor 1. J. Biol. Chem. 272:17327-17332. [DOI] [PubMed] [Google Scholar]
- 55.Yi, M., G. X. Tong, B. Murry, and C. R. Mendelson. 2001. Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1). J. Biol. Chem. 277:2997-3005. [DOI] [PubMed] [Google Scholar]
- 56.Young, P. P., and C. R. Mendelson. 1996. A CRE-like element plays an essential role in cAMP regulation of human SP-A2 gene in alveolar type II cells. Am. J. Physiol. 271:L287-L299. [DOI] [PubMed] [Google Scholar]
- 57.Young, P. P., and C. R. Mendelson. 1997. A GT box element is essential for basal and cyclic adenosine 3′,5′-monophosphate regulation of the human surfactant protein A2 gene in alveolar type II cells: evidence for the binding of lung nuclear factors distinct from Sp1. Mol. Endocrinol. 11:1082-1093. [DOI] [PubMed] [Google Scholar]
- 58.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]
- 59.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]