Abstract
Although sigma factor-dependent transcriptional regulation was shown to be essential for adaptation to different environmental stimuli, no such sigma factor has been related to the regulation of the cold shock response in Bacillus subtilis. In this study, we present genetic evidence for participation of σL (σ54) and the two σL-dependent transcriptional enhancers BkdR and YplP in the cold shock response of Bacillus subtilis JH642. Single-gene deletion of either sigL, bkdR, or yplP resulted in a cold-sensitive phenotype.
It has been shown that sigma factors allow the bacterial cell to regulate gene expression in response to different environmental stimuli (11, 14). The alternative sigma factor σB (12) is responsible for the induction of genes encoding general stress proteins following heat, ethanol, salt, and acid stress and during energy depletion in Bacillus subtilis (13). More than 150 general stress proteins or genes belong to the σB regulon (13), which provides nongrowing cells with a nonspecific, multiple, and preventive stress resistance (2, 10, 18). Interestingly, the sigB operon is also induced in cells continuously exposed to low temperatures (4) but not, however, after cold shock, which is defined as a sudden temperature shift from 37°C to 15°C (3). To explore the possible involvement of an alternative sigma factor in cold shock adaptation, we analyzed data from earlier transcriptional studies of B. subtilis (3). The cold-induced transcriptional regulator YplP was identified, which shares significant sequence similarity to σL-dependent transcriptional activators. The ΔyplP deletion mutant was shown to be cold sensitive in B. subtilis JH642 (3). The sigL gene encodes a homolog of the σ54 subunit of RNA polymerase and requires regulator proteins of the NtrC/NifA family to activate gene transcription (5, 9). Four homologs of the transcriptional regulator YplP in B. subtilis have been genetically characterized. AcoR, LevR, and RocR are involved in activation of acetoin, carbohydrate, and amino acid metabolism, respectively (1, 6, 8), while BkdR regulates the bkd operon. The strongly cold-induced bkd operon (16) is involved in the synthesis of precursor molecules for branched-chain fatty acids (7), which were shown to be essential for membrane adaptation after cold shock (3, 16). As both σL-dependent BkdR and YplP transcriptional regulators are linked to the cold shock response, we have investigated the role of σL in cold shock adaptation by monitoring the growth rates of ΔsigL, ΔbkdR, and ΔyplP deletion mutants after a sudden temperature shift from 37°C to 15°C.
Strain construction.
For the construction of strain ΔsigL (FW06) a DNA fragment was PCR amplified from chromosomal DNA of B. subtilis QB5505 (8) containing sigL disrupted with an aphA3 kanamycin resistance cassette with primers 5′sigL (TATTATCAAGGCTTTAGAGAGAAAATCGTC) and 3′sigL (ATGTTTTGTCAGCTCTTGTTTCAATGGCT). B. subtilis JH642 was transformed with the DNA fragment of 4,844 bp that was obtained, resulting in kanamycin-resistant strain ΔsigL (FW06).
For the construction of strain ΔbkdR (FW10), a DNA fragment was PCR amplified from chromosomal DNA of B. subtilis QB7512 (7) containing bkdR disrupted with an aphA3 kanamycin resistance cassette using primers 5′bkdR (ATTGCAACGGAATAAATAGGT) and 3′bkdR (ATGTTTGCGTTTATTCTGCAA). B. subtilis JH642 was transformed with the DNA fragment of 2,325 bp obtained, resulting in strain ΔbkdR (FW10). All strains used in this study are listed in Table 1.
TABLE 1.
B. subtilis strains in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| JH642 | pheA1 sfp0trpC2 | 15 |
| ΔyplP (CB15) | pheA1 sfp0trpC2 yplP::kan | 3 |
| ΔsigL (FW06) | pheA1 sfp0trpC2 sigL::aphA3 | This work |
| ΔbkdR (FW10) | pheA1 sfp0trpC2 bkdR::aphA3 | This work |
| ΔacoR (FW13) | pheA1 sfp0trpC2 acoR::aphA3 | This work |
| ΔlevR (FW14) | pheA1 sfp0trpC2 levR::aphA3 | This work |
| ΔrocR (FW15) | pheA1 sfp0trpC2 rocR::aphA3 | This work |
| ΔyplP2 (FW19) | pheA1 sfp0trpC2 yplP::kan amyE::yplP | This work |
| ΔyplP3 (FW20) | pheA1 sfp0trpC2 yplP::kan amyE::yplP yplQ | This work |
Growth analysis of JH642 deletion strains.
Prior to any further experiments, possible polar effects arising from the described gene deletions of yplP and bkdR were analyzed. The growth phenotype resulting from the deletion of yplP could be complemented in trans by introducing a copy of yplP in the amyE site under control of an inducible promoter (data not shown). The analogous experiment for the ΔbkdR mutant was described by Debarbouille et al. (7). Therefore, we conclude that the deletion of either yplP or bkdR does not have any polar effects.
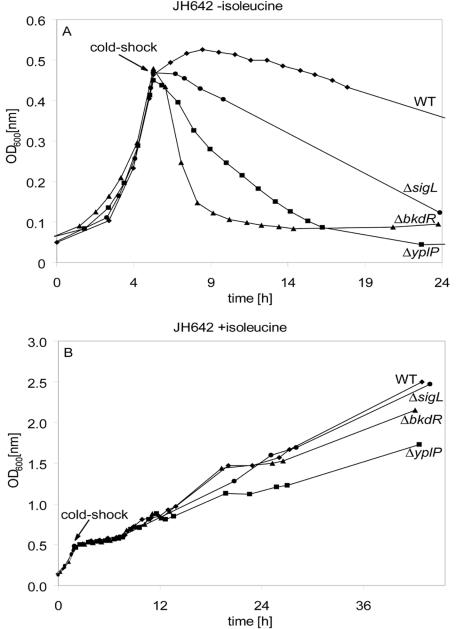
The deletion strains ΔbkdR (FW10) and ΔyplP (CB15) were grown in Spizizen's minimal medium (SMM) at 37°C and shocked to 15°C at an optical density at 600 nm (OD600) of 0.5 (Fig. 1A). Both ΔbkdR (FW10) and ΔyplP (CB15) lysed after cold shock, indicating that BkdR and YplP are important for the cold shock adaptation. In strain ΔbkdR (FW10), the transcriptional activator BkdR is not present any more to enhance the transcription of the bkd operon. Consequently, isoleucine is not converted to α-keto acids, and no branched-chain fatty acids are synthesized de novo to lower the melting point of the membrane (17). The cells lysed, due to the insufficient membrane adaptation in strain ΔbkdR (FW10). The observed lysis of ΔyplP (CB15) confirms the results of an earlier study (3); however, the underlying mechanism is still unknown.
FIG. 1.
Growth curves of B. subtilis JH642 (diamonds), ΔyplP (CB15) (squares), ΔbkdR (FW10) (triangles), and ΔsigL (FW06) (circles) in the absence (A) and presence (B) of isoleucine (50 μg/ml). Cells were grown in 200 ml SMM supplemented with 0.5% (wt/vol) glucose, 50 μg/ml tryptophan, 50-μg/ml phenylalanine, and trace elements at 37°C to an OD600 of 0.45 and then subjected to cold shock (15°C) (19). All experiments were repeated at least three times.
As both BkdR and YplP were shown to be important for cold shock adaptation, we investigated the role of the remaining three σL-dependent transcriptional activators, AcoR, LevR, and RocR. However, the analysis of the deletion mutant strains ΔacoR (FW13), ΔlevR (FW14), and ΔrocR (FW15) did not show cell lysis or cold-dependent growth retardation after a shift from 37°C to 15°C. This implies that AcoR, LevR, and RocR are not essential for cold shock adaptation (data not shown).
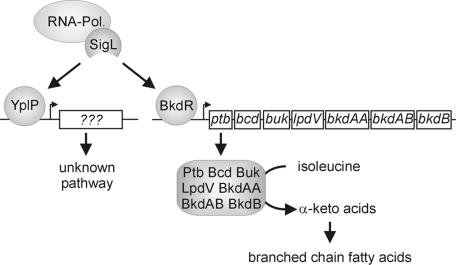
Αs σL interacted with the cold-relevant transcriptional regulators YplP and BkdR (Fig. 2), we examined its influence on cold shock adaptation. The deletion strain ΔsigL (FW06) was grown in SMM at 37°C and then shifted to 15°C at an OD600 of 0.5 (Fig. 1A). Strain ΔsigL (FW06) showed cell lysis after cold shock. This is the first demonstration that an alternative sigma factor is important for cold shock adaptation (Fig. 1A). However, the cold-sensitive phenotype of the ΔsigL mutant was not as severe as that observed for ΔbkdR and ΔyplP.
FIG. 2.
A diagram showing σL-dependent transcriptional enhancers BkdR and YplP, which are associated with cold shock adaptation and the downstream-regulated genes. The σL/BkdR regulation pathway is responsible for the cold shock adaptation of the membrane by the degradation of isoleucine to α-keto acids for branched-chain fatty acid synthesis. σL/YplP downstream-regulated cold-relevant genes are still unknown.
To evaluate if both transcriptional activators BkdR and YplP are involved in membrane adaptation, ΔsigL (FW06), ΔbkdR (FW10), and ΔyplP (CB15) were grown in SMM in the presence of isoleucine after a temperature shift from 37°C to 15°C (Fig. 1B). Under these conditions, the growth of all strains was improved after cold shock. While the addition of isoleucine supported the growth of ΔsigL (FW06) and ΔbkdR (FW10) to the level of the wild type, growth of ΔyplP (CB15) was significantly retarded (Fig. 1B). This suggests a membrane-dependent function of the σL/BkdR complex and a membrane-independent role of the σL/YplP complex for cold shock adaptation.
The σL/BkdR complex activates the bkd operon, which is responsible for the conversion of isoleucine to α-keto acids that are used as precursors for branched-chain fatty acid synthesis (Fig. 2). Although the transcriptional activator BkdR was missing in the ΔbkdR (FW10) mutant, sufficient amounts of α-keto acids were synthesized by residual amounts of the bkd operon-encoded enzymes if a large excess of isoleucine was present. This high substrate concentration compensated for the reduced amount of enzymes in the ΔbkdR (FW10) mutant. The residual amounts of bkd-encoded enzymes resulted from the basal transcription level of the bkd operon. Therefore, the observed growth rate of the ΔbkdR (FW10) mutant can be fully rescued by the addition of isoleucine. This is in full agreement with the published findings of Klein et al. (17), who showed that isoleucine serves as a switch in the fatty acid branching pattern for membrane adaptation to low temperatures. In contrast, the growth of the ΔyplP (CB15) mutant was not restored to wild-type levels. This suggests that the σL/YplP-regulated pathway contains at least one additional element, which is not involved in membrane adaptation.
We conclude that σL is involved in the regulation of at least two cold shock adaptation pathways in B. subtilis JH642 (Fig. 2). The first is the adaptation of the bacterial membrane by σL/BkdR-mediated activation of the bkd operon. The second is the σL/YplP-dependent pathway, with as-yet-unknown functions. Further investigations are in progress to reveal the nature of the σL/YplP-activated genes.
Acknowledgments
The Deutsche Forschungsgemeinschaft (SFB 395) supported this work.
We thank Julia Wiesner for technical assistance.
REFERENCES
- 1.Ali, N. O., J. Bignon, G. Rapoport, and M. Debarbouille. 2001. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 183:2497-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antelmann, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckering, C. L., L. Steil, M. H. Weber, U. Völker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brigulla, M., T. Hoffmann, A. Krisp, A. Volker, E. Bremer, and U. Volker. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck, M., M. T. Gallegos, D. J. Studholme, Y. Guo, and J. D. Gralla. 2000. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182:4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calogero, S., R. Gardan, P. Glaser, J. Schweizer, G. Rapoport, and M. Debarbouille. 1994. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J. Bacteriol. 176:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debarbouille, M., R. Gardan, M. Arnaud, and G. Rapoport. 1999. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debarbouille, M., I. Martin-Verstraete, A. Klier, and G. Rapoport. 1991. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both sigma 54- and phosphotransferase system-dependent regulators. Proc. Natl. Acad. Sci. USA 88:2212-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debarbouille, M., I. Martin-Verstraete, F. Kunst, and G. Rapoport. 1991. The Bacillus subtilis sigL gene encodes an equivalent of sigma 54 from gram-negative bacteria. Proc. Natl. Acad. Sci. USA 88:9092-9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelmann, S., and M. Hecker. 1996. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol. Lett. 145:63-69. [DOI] [PubMed] [Google Scholar]
- 11.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haldenwang, W. G., and R. Losick. 1980. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc. Natl. Acad. Sci. USA 77:7000-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecker, M., and U. Völker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 14.Helmann, J. D., and C. P. Moran. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 15.Hoch, J. A., and J. Mathews. 1973. Chromosomal location of pleiotropic sporulation mutations in Bacillus subtilis. Genetics 73:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaan, T., G. Homuth, U. Mader, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 17.Klein, W., M. H. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Völker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber, M. H., C. L. Beckering, and M. A. Marahiel. 2001. Complementation of cold shock proteins by translation initiation factor IF1 in vivo. J. Bacteriol. 183:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]