Abstract
The FsrABC system of Enterococcus faecalis controls the expression of gelatinase and a serine protease via a quorum-sensing mechanism, and recent studies suggest that the Fsr system may also regulate other genes important for virulence. To investigate the possibility that Fsr influences the expression of additional genes, we used transcriptional profiling, with microarrays based on the E. faecalis strain V583 sequence, to compare the E. faecalis strain OG1RF with its isogenic mutant, TX5266, an fsrB deletion mutant. We found that the presence of an intact fsrB influences expression of numerous genes throughout the growth phases tested, namely, late log to early stationary phase. In addition, the Fsr regulon is independent of the activity of the proteases, GelE and SprE, whose expression was confirmed to be activated at all three time points tested. While expression of some genes (i.e., ef1097 and ef0750 to -757, encoding hypothetical proteins) was activated in late log phase in OG1RF versus the fsrB deletion mutant, expression of ef1617 to -1634 (eut-pdu orthologues) was highly repressed by the presence of an intact Fsr at entry into stationary phase. This is the first time that Fsr has been characterized as a negative regulator. The newly recognized Fsr-regulated targets include other factors, besides gelatinase, described as important for biofilms (BopD), and genes predicted to encode the surface proteins EF0750 to -0757 and EF1097, along with proteins implicated in several metabolic pathways, indicating that the FsrABC system may be an important regulator in strain OG1RF, with both positive and negative effects.
Enterococcus faecalis is adapted to survive, persist, and proliferate in a wide range of environments as different as the gastrointestinal tract, heart valves, water, and soil. To do so, it is likely that E. faecalis has developed various mechanisms of adaptation. Examples include transcriptional regulators, such as hypR (47) or efaR (25); two-component systems (etaRS [46], croRS [9], vanSR [11], and RR1-13 [18]); and cell-cell signaling systems, including pheromone-inducible plasmid transfer (for a review, see reference 7), the Cyl system (8, 16), and the FsrABC system (30, 31, 34, 35).
The fsrABC operon, a homologue of agrABCD in Staphylococcus aureus, was originally shown by Qin et al. to activate, at the transcriptional level, the expression of two genes, gelE and sprE, coding for a metalloprotease and a serine protease, in addition to fsrBC (34, 35). Nakayama et al. and Qin et al. subsequently purified and characterized the FsrABC system pheromone as an 11-residue peptide lactone, produced from the C-terminal portion of the fsrB gene product and reaching peak levels at entry into stationary phase (ENT-stat) (30, 31, 35). Studies have also shown that, in the majority of the strains studied, a gelE+ genotype with a GelE− phenotype is associated with a 23.9-kb deletion, from ef1841 through part of ef1820 (ef1820 is fsrC). This deletion is found in many distinct clinical strains, as well as in isolates from healthy volunteers (32, 37). While Nakayama et al. found a correlation between the clinical origin of isolates and a Gel+ phenotype, Roberts et al., studying a larger panel of strains, did not see a statistically significant relationship (32, 37). Nonetheless, in the OG1RF background, the FsrABC system and gelatinase have been shown to be important for virulence in different animal models, with fsr and gelE mutants showing attenuation in a mouse peritonitis model (34), in Caenorhabditis elegans (43), in a rabbit endophthalmitis model (14, 29), in an Arabidopsis thaliana plant model (21), and, more recently, in an endocarditis model (44). Different outcomes have been observed in different assays when fsrABC or gelE-sprE mutants have been compared with the parental strain. In biofilm formation (18, 27) and translocation experiments (54), the double-protease mutant exhibits strong attenuation, and fsrABC mutants have not shown additional reduction. However, in the C. elegans model (43) and in the rabbit model of endophthalmitis (14, 29), an fsrBC mutant was more attenuated than a gelE-sprE mutant, indicating that the FsrABC system may regulate other genes important for virulence.
A number of groups have used microarray analysis to investigate complex regulatory cascades by taking snapshots during growth or by comparing strains. For example, in S. aureus, which shares a number of homologues with E. faecalis (e.g., Fsr and Agr [for a review, see reference 6] and Ace and Cna [53]), Dunman et al. described a complex regulatory network controlled by agr and sar (13), followed by studies looking at the rot (40) and sigB (3) regulons, all using transcriptional profiling. Recently, the targets of another quorum system, which was shown to regulate the expression of agr, were also examined using a microarray (23), thus adding another layer of complexity. However, much less is known about regulatory cascades in enterococci.
In this study, we present microarray-based data from three distinct genetic backgrounds (an fsrB in-frame deletion mutant, a gelE in-frame mutant, and a polar insertion mutation in gelE) compared to the parental strain, OG1RF. Our results show that the E. faecalis FsrABC system positively and negatively regulates the expression of numerous genes between late exponential growth phase and early stationary phase (EAR-stat). In addition, the FsrABC system regulon is independent of the expression of gelE and sprE.
MATERIALS AND METHODS
Bacterial strains and media.
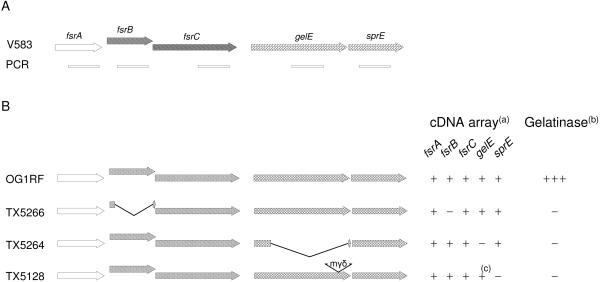
The strains used in this study were OG1RF (28) and its isogenic deletion mutants TX5266 (ΔfsrB) (35), TX5264 (ΔgelE) (43), and the gelE insertion mutant TX5128 (GelE− SprE−) (36). The details of the fsr-gel loci of these strains and the PCR fragments of these genes that were used for the array are shown in Fig. 1. Because of a chaining phenotype of TX5266 (unpublished data) and of TX5128 (48) compared to OG1RF, growth was assessed by the optical density at 600 nm and not by CFU. All strains were grown routinely in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) at 150 to 200 rpm or on BHI agar at 37°C with kanamycin (2 mg/ml) when needed for mutant selection.
FIG. 1.
Characterization of the strains used in this study. (A) Genetic organization of the fsr and gelE loci in V583 and locations of the PCR products of these genes used to construct the microarray. (B) Genotype and phenotype of the OG1RF parental and isogenic strains used in this study. TX5266 and TX5264 are both in-frame deletion mutants in which the deleted sequence contains the portion used for the microarray (fsrB and gelE, respectively). TX5128 is a gelE disruption mutant in which the minitransposon, mγδ, is inserted in the 3′ end of the gene. (a) Expression pattern using the V583 array. (b) Gelatinase activity. (c) Although the gene is truncated and the protein inactive, due to the location of the PCR primers, gelE transcript was detected in TX5128 by microarray.
RNA isolation and labeling.
RNA was extracted from cells grown in BHI broth with shaking at 37°C, conditions known to promote fsrB expression and used previously in investigations of the expression of fsrABC and gelE (34, 35). Briefly, after being cultured overnight, the cells were diluted so that the starting optical density at 600 nm was 0.05. Following 3 h (late log phase), 4 h (ENT-stat), and 5 h (EAR-stat) of incubation (Fig. 2A), cells were collected for RNA isolation. RNA was extracted from cultures using RNAwiz (Ambion, Austin, TX) according to the protocol provided by the supplier. Typically, between 15 and 30 μg of RNA was obtained per ml of culture. RNA was labeled with the FairPlay Microarray Labeling Kit (Stratagene, La Jolla, CA) using the manufacturer's protocol, with one exception. After the annealing step with poly(T), 1 μl of random primers (Invitrogen, Rockville, Md.) was added, and the sample was incubated again at 70°C for 10 min before being cooled on ice until it was ready to use. The appropriate samples, one labeled with Cy3 and the other with Cy5, were mixed and dried to completion using a speed vacuum.
FIG. 2.
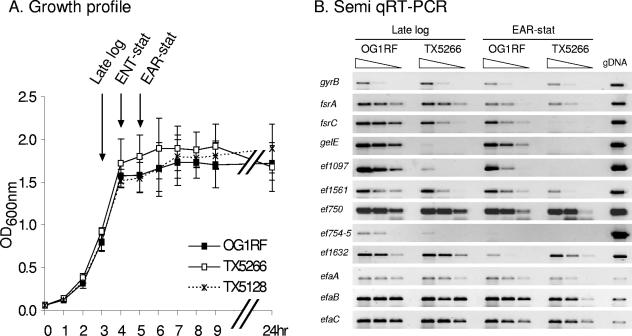
Growth profile of E. faecalis in BHI and semiquantitative RT-PCR. (A) The means and standard deviations were calculated from three independent cultures grown in parallel. OG1RF is the parental strain, while TX5266 is the in-frame fsrB deletion mutant, and TX5128 is a gelE disruption mutant. The arrows indicate when samples were taken for microarray analysis. Hour 3 corresponds to late log phase, hour 4 to ENT-stat, and hour 5 to EAR-stat. (B) Semiquantitative RT-PCR analysis of gyrB, fsrC, gelE, ef1097, ef1561, ef0750, ef754 to -755, ef1632, efaA, efaB, and efaC showing RT-PCR products from RNA extracted from OG1RF or TX5266 at hour 3 (late log phase), and hour 5 (EAR-stat). The three lanes for each RNA represent undiluted cDNA and two 10-fold dilutions of cDNA before the PCR. The gDNA used as a control for the PCR was extracted from OG1RF.
Slides and hybridization.
PCR products and slide processing were described by Aakra et al. (1). After being printed, the slides were rehydrated with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and dried using a heat block at 140°C for 15 s. Before use, the slides were prehybridized in 5× SSC, 0.1% sodium dodecyl sulfate (SDS), 1% bovine serum albumin for 1 h and washed with H2O and then with propanol before being dried using a centrifuge at 1,000 × g for 2 min. The dried probes were resuspended in 30-μl volumes (50% formamide, 5× SSC, 0.1% SDS, and 40 μg Cot1 DNA/ml). This mixture was heated for 10 min at 95°C, cooled on ice, and then added to a prehybridized slide under a coverslip. The slide was then placed at 42°C overnight in a sealed hybridization chamber (Corning 2551; Fisher Scientific Co., Pittsburgh, PA), which was humidified with 20 μl of 5× SSC. The arrays were then washed at room temperature, once in a solution of 2× SSC-0.1% SDS for 10 min, once in 0.1× SSC-0.1% SDS for 10 min, and once in 0.1× SSC for 2 min, followed by a final quick wash in double-distilled H2O.
The array was made with PCR products amplified with V583 genomic DNA (gDNA) using primers selected from the V583 genome sequence (33). To assess the percentage of genes detectable using the V583 array, we performed hybridization with gDNA from OG1RF and V583 using the 3DNA Array 900DNA kit (Genisphere, Hatfield, PA) as described in the manufacturer's protocol.
Data acquisition and statistics.
Hybridized microarray slides were scanned (GenePix Pro 5.0; Axon Instruments, Inc.) with independent excitation of the fluorophores Cy3 and Cy5 at 10-μm resolution. The signal and background fluorescence intensities were calculated for each DNA spot using the segmentation method of the GENPIXPRO software (Molecular Devices Corp., Union City, CA). After the local background of each spot intensity was subtracted, the ratios of intensities for Cy3- to Cy5-labeled probes were determined for each DNA spot.
To allow appropriate statistical analysis of the results, RNA preparations from at least three independent cultures were tested for each set of strains. For each hybridization, RNA was obtained from cultures of the OG1RF parent strain and the mutant strains grown in parallel. Each RNA preparation was used in at least two separate dye swap hybridizations (one with parent-Cy3/mutant-Cy5 and the other with parent-Cy5/mutant-Cy3).
The results for each mutant were analyzed separately, as described previously (4). After quantitation and global normalization using the average spot intensity, loge ratios of OG1RF to the ΔfsrB mutant, OG1RF to the ΔgelE mutant, or OG1RF to the gelE insertion mutant were calculated for each spot. For each open reading frame (ORF), loge ratios for each replicate culture were calculated by averaging loge ratios for spots (n = 5) within a chip that met quality criteria and their averaging dye-swap hybridizations. Unacceptable spots were those with no signal or those associated with a slide problem, such as dust or a scratch. ORFs were considered significantly regulated if (i) the overall change was at least twofold (i.e., the absolute value of the average loge ratio was greater than 0.693) and (ii) the P value from a one-sample t test, testing whether the grand mean loge ratio was different from 0.0, was significant at the 0.05 level or better.
Sequence analysis and results available online.
We used the following resources to characterize the predicted products of genes of interest: the KEGG website (http://www.KEGG.com), BLAST (http://www.ncbi.nlm.nih.gov/BLAST), SMART (http://smart.embl-heidelberg.de), TMpred (http://www.ch.embnet.org/software/TMPRED_form.html), and PredictProtein (http://www.embl-heidelberg.de/predictprotein/predictprotein.html).
A file containing the induction and P values for all regulated ORFs (>2-fold regulation; P < 0.05) from experiments comparing OG1RF to the TX5266 ΔfsrB strain is available in the supplemental material.
Semiquantitative RT-PCR.
RNA was extracted as described above from two independent cultures. Twenty micrograms of RNA was treated twice with DNase-Free solution (Ambion) according to the protocol of the supplier. For the reverse transcription (RT)-PCR, 10 μg of RNA was mixed with 2 μl of random decamers (Invitrogen; 50 μM) for a final volume of 18.5 μl in water. The mixture was heated to 70°C for 5 min and then immediately placed on ice. A master mixture comprised of 6 μl of 5× Superscript II buffer, 2 μl of deoxynucleoside triphosphate mixture (2.5 mM each), 3 μl of 0.1 M dithiothreitol, and 1 μl of Superscript II reverse transcriptase (Invitrogen; 200 U/μl) was added to the RNA solution (final volume, 30 μl), and the mixture was incubated at 42°C for 2 h. A control sample that contained RNA and all of the RT components except the Superscript II reverse transcriptase was prepared simultaneously. The PCRs were then performed using undiluted RNA control sample, serial dilution of cDNA (1, 1:10 and 1:100), and a gDNA template control. The list of primers is available in the supplemental material.
Mapping of ef1097 transcriptional start site.
Total RNA of E. faecalis OG1RF was isolated from late-log growth phase (3 h) and purified as described previously. The transcriptional start point of ef1097 was determined using the RACE 5′/3′ kit (Roche, Indianapolis, IN) according to the manufacturer's instructions.
RESULTS AND DISCUSSION
Overview of the Fsr regulon.
In this study, we quantified the genomewide transcriptional profile of OG1RF and its isogenic ΔfsrB mutant (Fig. 1) in three different growth phases, namely, late log phase (3 h after the cultures were started), at ENT-stat (4 h), and at EAR-stat (5 h) (Fig. 2A). It is known that in broth culture during this period, the FsrABC system regulates the gelE-sprE operon, which codes for gelatinase and serine protease, factors important for virulence, in addition to the fsrBC operon, by a quorum-sensing mechanism at the level of transcription. As shown in Fig. 3A, B, and C, four genes are markedly influenced by the deletion of fsrB in all three phases tested. In late log phase, expression of fsrB, fsrC, gelE, and sprE was, respectively, 480-, 44-, 725-, and 503-fold higher in OG1RF than in TX5266, the fsrB mutant. This increase in expression in the presence of an intact fsrB gene was maintained through early stationary phase with 118-, 33-, 215-, and 166-fold-enhanced transcription in the parental strain for the same genes. Considering the relative expression in the parent versus the fsrB mutant, we would classify all four genes as being fsrB dependent (the expression levels of these genes in the fsrB deletion mutant were below the detection level of the microarray). However, with undiluted cDNA and RT-PCR (Fig. 2B), the fsrC primers could be shown to amplify a product in TX5266 (in late log phase [3-h] and EAR-stat [5-h] cultures), confirming the basal expression presumably due, at least in part, to the fsrA promoter (35). In contrast, during these two phases, the fsrC product was detected with OG1RF cDNA diluted 1:100. Our data corroborate published studies by Qin et al. (35) showing that fsrBC is autoregulated and is critical for the expression of the gelE-sprE operon, while fsrA is constitutively expressed at a low level (detected by microarray in all three phases), but neither fsrB nor growth phase regulated.
FIG. 3.
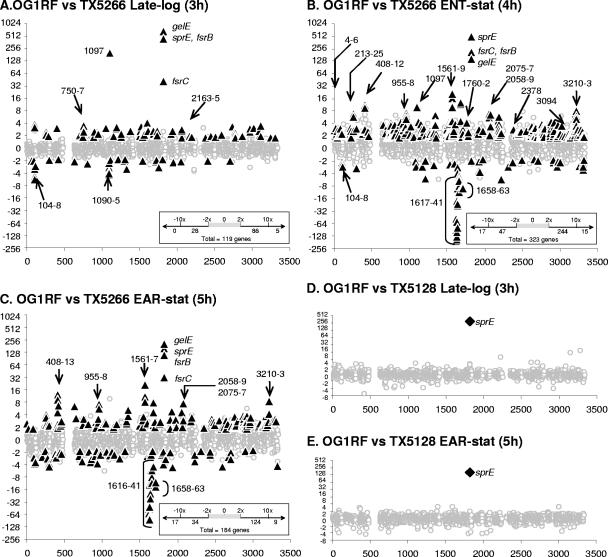
Alteration in gene expression between OG1RF and TX5266 (fsrB in-frame deletion) and between OG1RF and TX5128 (gelE disruption mutant). Each panel represents an average of microarray results obtained with RNA preparations from at least three different cultures. The genes are represented if at least one average ratio per RNA preparation was available for statistical analysis. For all panels, the x axis represents the gene identification number as annotated by TIGR (NCBI ID, NC_004668). The y axis indicates the change (n-fold); the change was considered positive when the expression was higher in OG1RF than in the mutant. The gray circles correspond to nonsignificantly affected genes. The black triangles (A, B, and C) correspond to genes that were regulated at least twofold with a P value of <0.05 for OG1RF versus TX5266, while the black diamonds (D and E) represent genes that were regulated at least twofold with a P value of <0.05 for OG1RF versus TX5128. The arrows indicate specific genes of interest (“ef” has been omitted). In the boxes in the right lower corners of panels A to C are shown the numbers of regulated genes and their classification into four categories: >10-fold repression, between 2- and 10-fold repression, between 2- and 10-fold activation, and >10-fold activation.
At least two published studies have suggested that the regulon of the FsrABC system should be greater than just activation of the gelE-sprE operon; that is, an fsrB mutant was more attenuated than a gelE-sprE double mutant in a C. elegans model (43) and in a rabbit model of endophthalmitis (14, 29). Consistent with these results, we found, in late log phase, that 119 genes were affected by the deletion of fsrB (at least twofold difference in expression between OG1RF and TX5266; P < 0.05), although only one additional gene was found to be fsrB dependent, ef1097 (214×; P = 0.004) (Fig. 3A), while others generally showed a two- to sixfold difference (see the supplemental material). At entry into stationary phase (Fig. 3B), 323 genes were statistically significantly regulated, 259 activated, and 64 repressed by the Fsr system. One set of genes, activated two- to threefold, encodes proteins likely to be growth phase dependent, suggesting the possibility that, although the growth curves appear similar between OG1RF and TX5266 (Fig. 2A), slight differences in growth profile may have led to statistically significant gene expression differences, as described by Sapolsky in his chronotranscriptome study of Bacillus subtilis (41). In EAR-stat (5 h), these putatively growth phase-dependent genes were no longer fsrB regulated (Fig. 3B and C). However, 109 genes remained fsrB regulated in EAR-stat, with an additional 76 new genes also showing evidence of fsrB regulation (Fig. 3C). Among these 185 genes, the expression levels of 9 were >10-fold higher in OG1RF than in TX5266. These genes belong to three loci: the fsrABC-gelE-sprE locus, four genes of the ef1561 to -1567 operon (with an average induction for all genes of ca. 13-fold), and ef0411, a member of the ef0408 to -0413 operon (with an average induction of 10-fold). On the other hand, the expression of 17 genes increased >10-fold in the absence of fsrB (TX5266) compared to OG1RF. Among these 17 genes, 14 showed no detectable expression in the parent strain, while high levels of expression were detected in the mutant (homologues of the eut-pdu genes [ef1617 to -1635]) (Fig. 2B and 3C). This is the first time that the FsrABC system has been suggested as a negative regulator, although whether this is a direct or indirect effect remains to be elucidated.
The Fsr regulon is independent of gelatinase activity.
The FsrABC system has been recognized as the gelE-sprE regulator. The gelE-sprE operon codes for proteases that, once secreted and activated, may have a number of effects that could cause altered cell physiology; for example, a gelE disruption mutant of OG1RF is known to show extensive chaining (48). It is also possible that proteases might release or degrade a second autoinducer, and this might be responsible for some of the differences seen between OG1RF and the fsr mutants, particularly in EAR-stat. To examine this possibility, we compared the transcriptome of OG1RF with TX5264, a gelE deletion mutant, at two different growth phases (late log phase and EAR-stat). No difference was observed, except for gelE itself (the deletion includes the sequence of the PCR fragment used for the array) (Fig. 1B). Since Kawalec et al. (22) showed that absence of gelatinase activity results in aberrant processing of pro-SprE and the appearance of a “superactive” form of the enzyme, we also examined the gelE disruption mutant, TX5128 (GelE− SprE−) in these two growth phases (late log phase and EAR-stat). As shown in Fig. 3D and E, no gene was significantly affected (as defined by a ratio of >2 and a P value of <0.05) in the disruption mutant (TX5128) versus OG1RF between late log phase and EAR-stat, except the sprE gene, which showed a >200-fold (P < 0.05) decrease in TX5128 compared to OG1RF. These results are consistent with those of Qin et al., who found that sprE is not expressed in a gelE insertion mutant due to the downstream polar effect of the insertion (35). The gelE mRNA level is not altered in the microarray analysis, because the gelE primers used to create the array are upstream of the disruption (Fig. 1A and B). When OG1RF and TX5128 were compared, the variance of the ratio of nonsignificantly affected genes (Fig. 3) was 0.078 at 3 h (late log phase) and 0.071 5 h (EAR-stat), confirming that there are few differences in the expression of other genes between the parent and the GelE− SprE− mutant. In contrast, the variance ranged from 0.069 in late log phase to 0.213 in ENT-stat and 0.200 in EAR-stat when OG1RF was compared with TX5266, the ΔfsrB mutant.
The importance of the proteases in virulence has been proven in different models, even if the mechanism is not well understood. Our microarray results imply that (i) the Fsr regulon is not controlled via a mechanism dependent on GelE and/or SprE activity under our conditions and (ii) the lack of protease activity does not lead to significant changes in gene expression by microarray analysis in the two growth phases studied. These results, albeit not performed with in vivo-grown organisms, suggest that the decrease in virulence seen with a gelE-sprE mutant strain is not due to a secondary effect on the expression of other genes. Additional studies will be needed to characterize the targets of GelE and SprE and their specific effects on biofilm formation and translocation and in heart valves and/or vegetations.
Influence of Fsr on regulatory cascades.
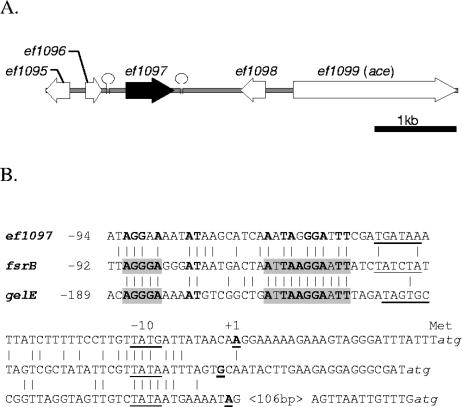
Qin et al. characterized a consensus sequence necessary for expression of the fsrBC and gelE-sprE operons in their promoter area (35). Using in silico analysis with the sequence [ATCG]AGG[GA]A[AG]\w {13 to 16 bp}[ATCG]AAGGA[AG], we found eight additional representations of this consensus sequence in front of potential ORFs (ef0126, ef0138, ef0198, ef0562, ef1839, ef1890, ef2702, and ef3132). None of these genes appeared to be affected by the fsrB deletion under the conditions used. In addition to fsrBC and gelE-sprE, ef1097 (ef1097 is described below) was the only other gene that was also fsrB dependent for its expression (i.e., no detectable expression in TX5266, while expression was present in OG1RF) (Fig. 3A and B). We determined the transcriptional start point of ef1097 by 5′ rapid amplification of cDNA ends-PCR and found that the A that is 24 bp upstream of the ATG codon corresponds to the +1 of the transcript. An alignment between gelE, fsrB, and ef1097 (Fig. 4B) shows that the ef1097 promoter area differs slightly from the previously predicted consensus sequence. A less stringent consensus sequence (AGG[AG]{17 bp}A[AG]GGA) was not found upstream of other fsrB-regulated genes. Now that we have a better understanding of the extent of the Fsr regulon, additional work will be needed to define the fsrB consensus sequence and what other elements are required for the other highly fsrB-regulated genes.
FIG. 4.
ef1097 locus and transcriptional start site. (A) Genetic organization of the ef1097 chromosomal region. (B) Sequence alignment between ef1097, gelE, and fsrB promoter regions. Putative −10 and −35 sequences are underlined, and transcription start sites (+1) are boldface and underlined. The two boxes characterized by Qin et al. (36) as essential for expression are shaded. Common nucleotides between ef1097, fsrB, and gelE promoters are boldface. The stem loops indicate a putative transcriptional terminator.
As potential candidates for members of secondary regulation pathways, we found that 25 homologues of known regulatory proteins (identified by The Institute for Genomic Research [TIGR] annotation) that were fsrB regulated throughout the different growth phases listed in Table 1. In late log phase, four genes coding for potential regulatory proteins showed higher expression in OG1RF versus TX5266, while two genes were less expressed in the parental strain than in the fsrB mutant. At ENT- and EAR-stat, expression of two genes, ef1632 and ef1633, could not be detected in OG1RF, while they were expressed at a high level by TX5266. These two genes appear to code for a putative two-component system, of which EF1632 would be the histidine kinase and EF1633 the response regulator (HK17 and RR17) (17). These two genes are included in the eut-pdu locus. In Salmonella enterica, this locus encodes enzymes necessary for the transport and degradation of phosphatidylethanolamine, and expression of the eut-pdu operon is activated by eutR in response to the simultaneous presence of ethanolamine plus adenosylcobalamin (38, 42). In the V583 genome, no homologue was found for eutR. It should be of interest to see if EF1632 and EF1633 share a similar function with EutR of S. enterica. Inactivation of EF1633, also known as HK17, in the JH2-2 background did not show an effect on antibiotic resistance, biofilm formation, or environmental stress (including osmolarity, oxidative stress, low pH, heat, and detergents) (19). On the other hand, this work was done in a JH2-2 background, a strain known as a natural fsrB mutant due to a 23.9-kb deletion (32).
TABLE 1.
Regulatory proteins influenced by fsrB deletion
| IDa | Putative function | Late log
|
ENT-stat
|
EAR-stat
|
|||
|---|---|---|---|---|---|---|---|
| Change (n-fold)b | Pc | Change (n-fold) | P | Change (n-fold) | P | ||
| EF0097 | Regulatory protein (PfoR family) | 4 | 0.05 | ||||
| EF0301 | Transcriptional regulator (GntR family) | −2 | 0.02 | ||||
| EF0372 | RR13d | 2 | 0.02 | ||||
| EF0927 | HX09d | −2 | 0.02 | ||||
| EF1051 | HK10d | 3 | <0.01 | ||||
| EF1306 | Heat-inducible transcription repressor HrcA | 2 | <0.01 | ||||
| EF1515 | Transcription antiterminator BglG | 3 | <0.01 | ||||
| EF1522 | RNA polymerase sigma-70 factor | 3 | 0.01 | ||||
| EF1525 | Ferric uptake regulator | 2 | 0.02 | ||||
| EF1569 | Transcriptional regulator; PSR protein | 5 | 0.02 | ||||
| EF1632 | HX17d | −18 | 0.01 | −12 | 0.01 | ||
| EF1633 | RR17d | −24 | 0.01 | −21 | <0.01 | ||
| EF2063 | Transcriptional activator (AraC family) | 5 | 0.04 | ||||
| EF2218 | RR01d | 2 | 0.02 | ||||
| EF2417 | Ferric uptake regulator | 2 | 0.05 | ||||
| EF2426 | Transcriptional regulator (GntR family) | 2 | 0.01 | ||||
| EF2703 | Transcriptional regulator | −2 | 0.03 | ||||
| EF2711 | Transcriptional regulator (AraC family) | −2 | 0.04 | −3 | 0.02 | ||
| EF2767 | Transcriptional regulator | 2 | 0.01 | 3 | <0.01 | ||
| EF2911 | DNA-binding response regulator | 2 | 0.02 | 2 | 0.04 | ||
| EF3196 | RR02d | 2 | 0.01 | ||||
| EF3214 | Sigma-54-dependent transcriptional regulator | 4 | <0.01 | ||||
| EF3216 | Transcriptional regulator; putative | 3 | 0.02 | ||||
| EF3308 | Transcriptional activator | −2 | 0.02 | ||||
| EF3309 | Transcriptional antiterminator (BglG family) | −3 | 0.01 | ||||
EF numbers and their putative functions are from the V583 genome sequenced by TIGR (NCBI ID, NC_004668).
The change represents mRNA expression levels in OG1RF relative to those in the fsrB mutant at specific growth phase time points and corresponds to averages of experiments done with three independent cultures. Minus indicates that the expression was lower in the wild type than in the fsrB mutant (TX5266).
The P value from a one-sample t test, testing whether the grand mean loge ratio was different from 0.0, was significant at the 0.05 or better level for each of the genes tested.
HK, histidine kinase; RR, response regulator (17).
The fact that Fsr regulates genes coding for potential regulatory proteins may explain why we found fewer fsrB-regulated genes in late log phase than were observed at the later time points (ENT- and EAR-stat); that is, the increase in regulated genes may be, to some extent, the consequence of secondary regulatory cascades. Agr in S. aureus, which is in the same family of regulators as Fsr, shares some regulatory pathways with other regulators, like SarA, but also directly modulates the expression of other regulatory proteins, such as PyrR (13).
The FsrABC system is an important regulator of “food” supplies.
As would be expected with a quorum-sensing system, the Fsr regulon changes dramatically with entry into stationary phase. As can be seen in Fig. 3B and C and in Table 2, there is a cluster of genes that was found to be highly repressed in the presence of fsrB upon entry into stationary phase (4 h) and that remained repressed in early stationary phase (5 h). These genes can be divided into two sets. One set corresponds to ef1617 to ef1638 (eut-pdu orthologues); as mentioned earlier, the expression of these genes was noted to be turned off at ENT-stat by the presence of an intact fsrB (Fig. 2B). For the second set of genes (ef1658 to -1663), expression was only partially repressed in the parent strain. However, the level of regulation remained substantial, with a 5- to 11-fold decrease for ef1658 to -1663 expression in OG1RF compared to TX5266 at ENT- and EAR-stat.
TABLE 2.
Metabolic pathways regulated by Fsr
| IDa | Gene name or definitionb | Changec
|
||
|---|---|---|---|---|
| Late log | ENT-stat | EAR-stat | ||
| Arginine and proline metabolism | ||||
| EF0104 | arcA | −5 | −3 | |
| EF0105 | argF-1 | −6 | −2 | |
| EF0106 | arcC-1 | −3 | −3 | −4 |
| PTS system | ||||
| EF0411 | Mannitol-specific IIBC components | 12 | 13 | |
| EF0412 | mltF | 10 | 9 | |
| EF0413 | mtlD | 7 | ||
| EF0955 | bopC/malMd | 6 | ||
| EF0956 | bopB/malB | 7 | 6 | |
| EF0957 | bopA/malP | 6 | 7 | |
| EF0958 | mall | 5 | 6 | |
| EF3210 | Putative PTS system, IIA component | 7 | 3 | |
| EF3211 | PTS system, IIB component | 8 | 9 | |
| EF3212 | PTS system, IIC component | 6 | 5 | |
| EF3213 | PTS system, IID component | 5 | 4 | |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | ||||
| EF1561 | aroE | 5 | 9 | |
| EF1562 | P-2-dehydro-3-deoxyheptonate aldolas | 2 | 17 | 24 |
| EF1563 | aroB | 3 | 24 | 22 |
| EF1564 | aroC | 2 | 21 | 9 |
| EF1565 | Prephenate dehydrogenase | 2 | 11 | 13 |
| EF1566 | aroA | 11 | 11 | |
| EF1567 | aroK | 4 | 8 | |
| EF1568 | Prephenate dehydratase | 10 | ||
| EF1569 | psr | 5 | ||
| Glycerophospholipid metabolism | ||||
| EF1616 | CoA-binding domain protein | −2 | ||
| EF1617 | Hypothetical protein | −87 | −49 | |
| EF1618 | eutH | −63 | −32 | |
| EF1619 | Putative pduN | −51 | −59 | |
| EF1620 | Hypothetical protein | −95 | −63 | |
| EF1621 | Hypothetical protein | −96 | −92 | |
| EF1622 | Hypothetical protein | −181 | −45 | |
| EF1623 | Microcompartment protein | −61 | −45 | |
| EF1624 | Putative aldehyde dehydrogenase | −165 | −77 | |
| EF1625 | Microcompartment protein family | −8 | −6 | |
| EF1627 | eutC | −139 | −77 | |
| EF1629 | eutB | −288 | −86 | |
| EF1630 | Chaperonin? (frameshift) | −107 | −43 | |
| EF1632 | Sensor nistidine kinase | −18 | −12 | |
| EF1633 | Response regulator | −24 | −21 | |
| EF1634 | eutS | −85 | −27 | |
| EF1635 | Putative pduU | −18 | −7 | |
| EF1637 | ATP:cob(I)alamin adenosyltransferase | −9 | ||
| EF1638 | pduv | −22 | ||
| Valine, leucine, and isoleucine degradation | ||||
| EF1658 | bkdC | −5 | ||
| EF1659 | bkdB | −7 | −8 | |
| EF1660 | bkdA | −8 | −11 | |
| EF1661 | bkdD | −6 | ||
| EF1662 | buk | −11 | ||
| EF1663 | ptb | −6 | −6 | |
Only those operons in which at least one gene was at least fivefold Fsr regulated and where a metabolic function was attributed using the KEGG database are represented. EF numbers and their putative functions are from the V583 genome sequenced by TIGR (NCBI ID, NC_004668).
The gene name or definition was obtained using the KEGG database.
See note b in Table 1; in all cases, the P value was <0.05.
In Salmonella, use of ethanolamine as a carbon and nitrogen source may be important, since this compound is a constituent of an abundant species of lipid present in the intestinal tract: phosphatidylethanolamine (51). In S. enterica, the eut/pdu operon contains the genes encoding the ethanolamine metabolosome: the complex containing the enzymatic machinery necessary for the degradation of ethanolamine to acetyl-coenzyme A (CoA) (5). Based on the KEGG database and BLAST searches, it seems that OG1RF carries all the genes needed for the expression of the ethanolamine metabolosome in its equivalent of the eut/pdu operon, namely, ef1617 to ef1634, which appeared to be highly expressed at late log phase by both the parental and the fsrB mutant strains. At ENT-stat and EAR-stat, the FsrABC system appeared to turn off the eut/pdu operon, suggesting the possibility that, when detecting a limitation for some essential nutrients (glycerophospholipid), OG1RF cells redirect their metabolism to avoid the use of phosphatidylethanolamine as a carbon and nitrogen source.
Following the same theme, we observed enhanced transcription (4- to 24-fold) of ef1561 to -1567 in OG1RF versus TX5266 from ENT-stat (Fig. 3B and C and Table 2), also seen by semiquantitative RT-PCR (Fig. 2B). This operon codes for enzymes predicted to be necessary for phenylalanine and tyrosine synthesis. This regulation profile is reversed in the case of ef1658 to -1663, genes coding for enzymes implicated in isoleucine, valine, and leucine degradation pathways (reduced transcription, at ENT-stat, by 5- to 11-fold in OG1RF versus TX5266 for all the genes of the operon and 8- to 11-fold for ef1659 to -1660 and ef1663 at EAR-stat). The repression of the eut-pdu operon, described above, should also lead to the conservation of methionine by reducing its use as a methyl donor. It has been shown that methionine, tryptophan, histidine, and isoleucine are essential for all the E. faecalis strains tested, while arginine, glutamate, glycine, leucine, and valine are important for OG1RF yet appear to be essential for JH2-2 (28). We can postulate that strain JH2-2, a natural fsrB mutant, is more sensitive to the absence of some or all of these amino acids than OG1RF because its FsrABC system is not functional to protect at least leucine and valine from degradation.
Finally, at both ENT-stat and EAR-stat, the expression of three phosphotransferase (PTS) systems was activated in the presence of fsrB (ef0408 to -0413 [∼9-fold], ef0955 to -0958 [∼6-fold], and ef3210 to -3213 [∼7-fold]). Based on the KEGG website, each of these PTS systems appears to be sugar specific, with EF408 to -412 specific for mannitol, EF0955 to -0958 for maltose, and EF3210 to -3213 for mannose. Interestingly, the ef0954 to -0958 locus has been described in two different papers: (i) as bopABCD (ef0957 to -0954) (20) and (ii) as malT (ef0958) and malPBMR (ef0957 to -0954) (24). Both malT (ef0958) and malP (ef0957/bopA) appear to be essential for maltose transport and utilization in strain JH2-2 (24) and, using E. faecalis type 9 strain, bopD (ef0954/malR, a LacI family transcriptional regulator), but not bopABC, appears to be important for biofilm formation and for bacteremia in mice (20). These data suggest that Fsr plays a positive role in biofilm production by at least two independent mechanisms: through the activation of gelatinase production (18, 27) and through the activation of bopABCD expression (20).
Factors (potentially) important for virulence.
In addition to GelE and SprE, we also found that the FsrABC system of OG1RF regulates at least one other well-described factor important for OG1RF virulence, namely, EfaA. EfaA is a 37-kDa dominant antigen in infective endocarditis caused by E. faecalis (2, 26) and is part of what is predicted to be an ABC-type transporter, with EfaA being the lipoprotein component. efaA is the third gene of the efaCBA operon. efaB (ef2075) and efaA (ef2076) expression was activated in OG1RF three- to eightfold (P < 0.05) compared to the fsrB mutant in ENT-stat and EAR-stat (Fig. 2B and 3B and C), while no statistically significant results were obtained for the first gene (ef2074). All three genes were expressed, but not Fsr regulated, in late log phase. In strain JH2-2, expression of this operon is Mn+ dependent and regulated via EfaR, a DNA binding protein (25). In OG1RF, the Fsr effect on efaA and efaB expression appeared to be independent of the expression of efaR, because although expressed, efaR was not regulated under any of our conditions. It would be of interest to investigate if another regulator of JH2-2 (which lacks fsrABC) influences the expression of genes that are part of the Fsr regulon of OG1RF, similar to what was shown recently in Pseudomonas aeruginosa, where the regulon of MvfR modulates some of the lasRI/rhlRI-regulated genes without directly affecting the lasRI or rhlRI systems (12).
The FsrABC system also regulates ef2058 to -2059, coding for orthologues of cydC and cydD (components of an ABC-type transporter required for assembly of cytochrome bd in Escherichia coli [10] and in Bacillus subtilis [52]). In Shigella flexneri (49, 50), as in Brucella abortus (39), this ABC transporter is critical for intracellular survival and full bacterial virulence. ef2058 to -2059 are part of an operon (ef2061 to -2058) in which the expression level was constant for the two first genes of the operon (ef2061 and -2060) while the expression level of the two downstream genes (ef2059 and -2058) was affected in the Fsr mutant as early as late log phase. These two genes were expressed threefold higher in late log phase and six- to sevenfold higher at ENT-stat, and at EAR-stat, cydD was expressed fivefold higher in the parent strain than in TX5266 (Fig. 3B and C). As with the efa operon, this is the second time that only the downstream genes of an operon appeared to be Fsr activated. We can postulate that the Fsr system may have an effect on mRNA stability.
As mentioned previously and as shown in Fig. 2B, only one other gene besides the fsr and gel loci was dependent on the presence of an intact fsrB gene for its expression in late log phase to ENT-stat: ef1097. In OG1RF, expression of ef1097 was very high in late log phase, decreased significantly at ENT-stat (4 h), and finally was undetectable at EAR-stat (5 h) by microarray. This gene is localized 2 kb upstream of ef1099 (Fig. 4A), a gene encoding Ace, a well-characterized collagen adhesin (32). ef1097, like ace, is present in clinical as well as in food isolates (P. Serror, personal communication). The gene codes for a putative membrane protein (170 amino acids) of unknown function that shares some homology with uncharacterized plasmid proteins from Streptococcus pyogenes (42% identity; 65% similarity) and Corynebacterium jeikeium (25% identity; 50% similarity). As with the ef0750 insertion mutant, no obvious difference in growth rate or in cellular physiology was observed when an ef1097 insertion mutant was compared with OG1RF in BHI broth culture, and no difference in pathogenicity in the C. elegans worm model was observed (D. Garsin, personal communication).
Of interest, too, is an operon including eight genes, ef0750 to -0757, that was found to be activated in the presence of an intact fsrB by two- to fourfold (P < 0.01) in late log phase and at ENT-stat. The level of ef0750 to -0757 expression decreased between late log phase and ENT-stat, although the level of regulation of the genes with detectable intensity remained stable (∼4-fold). At EAR-stat, the level of expression reached an undetectable level with each strain by microarray, but ef0750 expression was still detected by RT-PCR. The expression of ef0755 to -0757 compared to ef0750 to -0754 was 10 times lower, likely due to the presence of a weak termination loop (Fig. 2B and data not shown). In the V583 genome, five operons were found with an organization similar to that of ef0750 to -0757. Two of these five operons were found to be expressed in OG1RF, although only the ef0750 to -0757 operon was regulated by the FsrABC system under our conditions. The presence of the fsrB-regulated complex ef0750 to -0757, encoding surface and secreted proteins, with at least four paralogous systems in E. faecalis but none in other species, is intriguing.
Conclusion.
The Fsr system, known to be important for virulence in several animal models, is critical for expression of the gelE-sprE operon. In this study, we characterized the Fsr regulon in broth culture in three successive phases of growth (late log phase, at entry into stationary phase, and 1 h after entry into stationary phase). Although we were unable to study all OG1RF genes (10 to 15% of the OG1RF genes identified in reference 15 are not present in the V583 genome, and only 75% of the V583 PCR products printed on the array appeared positive with OG1RF genomic DNA) (data not shown), the extent of the Fsr regulon establishes that the FsrABC system is an important general regulator in OG1RF. From the other Agr-like systems studied, it appears that Agr-like systems act as significant regulators in pathogenic species (Agr in S. aureus and now Fsr in E. faecalis) and as limited regulators in nonpathogenic organisms (Lam in Lactobacillus plantarum) (45).
Fsr activates and represses numerous genes: besides gelE and sprE, the strongest activation effect (undetectable expression in the mutant) was seen in late log phase with ef1097, which encodes a putative membrane protein of unknown function, while the strongest repression effect (undetectable expression in the parent) was seen at ENT-stat and EAR-stat with ef1617 to -1638 (eut and pdu locus). New findings concerning fsrB-regulated factors potentially important for virulence, such as ef2058 to -2059 (cydCD), ef0954 to -0957 (bopABCD), or ef0750 to -0757 (putative membrane complex), may help elucidate additional pathogenicity mechanisms independent of the function of GelE and SprE of E. faecalis.
Supplementary Material
Acknowledgments
We are grateful to Xiang Qin for his help in bioinformatics and to Danielle Garsin for her help with the C. elegans worm model.
The present work was supported by grant NIH R37 AI47923 from the Division of Microbiology and Infectious Diseases, NIAID, to B.E.M.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aakra, A., H. Vebo, L. Snipen, H. Hirt, A. Aastveit, V. Kapur, G. Dunny, B. Murray, and I. F. Nes. 2005. Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob. Agents Chemother. 49:2246-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitchison, E. J., P. A. Lambert, and I. D. Farrell. 1986. Antigenic composition of an endocarditis-associated isolate of Streptococcus faecalis and identification of its glycoprotein antigens by ligand blotting with lectins. J. Med. Microbiol. 21:161-167. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bachi, and S. Projan. 2004. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J. Bacteriol. 186:4085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgogne, A., M. Drysdale, S. G. Hilsenbeck, S. N. Peterson, and T. M. Koehler. 2003. Global effects of virulence gene regulators in a Bacillus anthracis strain with both virulence plasmids. Infect. Immun. 71:2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinsmade, S. R., T. Paldon, and J. C. Escalante-Semerena. 2005. Minimal functions and physiological conditions required for growth of Salmonella enterica on ethanolamine in the absence of the metabolosome. J. Bacteriol. 187:8039-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner, S., H. Monteil, and G. Prevost. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 28:183-200. [DOI] [PubMed] [Google Scholar]
- 7.Clewell, D. B., and G. M. Dunny. 2002. The Enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, D.C.
- 8.Coburn, P. S., C. M. Pillar, B. D. Jett, W. Haas, and M. S. Gilmore. 2004. Enterococcus faecalis senses target cells and in response expresses cytolysin. Science 306:2270-2272. [DOI] [PubMed] [Google Scholar]
- 9.Comenge, Y., R. Quintiliani, Jr., L. Li, L. Dubost, J. P. Brouard, J. E. Hugonnet, and M. Arthur. 2003. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 185:7184-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Ramos, H., G. M. Cook, G. Wu, M. W. Cleeter, and R. K. Poole. 2004. Membrane topology and mutational analysis of Escherichia coli CydDC, an ABC-type cysteine exporter required for cytochrome assembly. Microbiology 150:3415-3427. [DOI] [PubMed] [Google Scholar]
- 11.Depardieu, F., B. Perichon, and P. Courvalin. 2004. Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J. Clin. Microbiol. 42:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deziel, E., S. Gopalan, A. P. Tampakaki, F. Lepine, K. E. Padfield, M. Saucier, G. Xiao, and L. G. Rahme. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-l-homoserine lactones. Mol. Microbiol. 55:998-1014. [DOI] [PubMed] [Google Scholar]
- 13.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelbert, M., E. Mylonakis, F. M. Ausubel, S. B. Calderwood, and M. S. Gilmore. 2004. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect. Immun. 72:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garsin, D. A., J. Urbach, J. C. Huguet-Tapia, J. E. Peters, and F. M. Ausubel. 2004. Construction of an Enterococcus faecalis Tn917-mediated-gene-disruption library offers insight into Tn917 insertion patterns. J. Bacteriol. 186:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, L., and M. Perego. 2002. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 184:5819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, L. E., and M. Perego. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 186:5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, L. E., and M. Perego. 2004. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J. Bacteriol. 186:7951-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hufnagel, M., S. Koch, R. Creti, L. Baldassarri, and J. Huebner. 2004. A putative sugar-binding transcriptional regulator in a novel gene locus in Enterococcus faecalis contributes to production of biofilm and prolonged bacteremia in mice. J. Infect. Dis. 189:420-430. [DOI] [PubMed] [Google Scholar]
- 21.Jha, A. K., H. P. Bais, and J. M. Vivanco. 2005. Enterococcus faecalis mammalian virulence-related factors exhibit potent pathogenicity in the Arabidopsis thaliana plant model. Infect. Immun. 73:464-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawalec, M., J. Potempa, J. L. Moon, J. Travis, and B. E. Murray. 2005. Molecular diversity of a putative virulence factor: purification and characterization of isoforms of an extracellular serine glutamyl endopeptidase of Enterococcus faecalis with different enzymatic activities. J. Bacteriol. 187:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korem, M., Y. Gov, M. D. Kiran, and N. Balaban. 2005. Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence. Infect. Immun. 73:6220-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Breton, Y., V. Pichereau, N. Sauvageot, Y. Auffray, and A. Rince. 2005. Maltose utilization in Enterococcus faecalis. J. Appl. Microbiol. 98:806-813. [DOI] [PubMed] [Google Scholar]
- 25.Low, Y. L., N. S. Jakubovics, J. C. Flatman, H. F. Jenkinson, and A. W. Smith. 2003. Manganese-dependent regulation of the endocarditis-associated virulence factor EfaA of Enterococcus faecalis. J. Med. Microbiol. 52:113-119. [DOI] [PubMed] [Google Scholar]
- 26.Lowe, A. M., P. A. Lambert, and A. W. Smith. 1995. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infect. Immun. 63:703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed, J. A., W. Huang, S. R. Nallapareddy, F. Teng, and B. E. Murray. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mylonakis, E., M. Engelbert, X. Qin, C. D. Sifri, B. E. Murray, F. M. Ausubel, M. S. Gilmore, and S. B. Calderwood. 2002. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect. Immun. 70:4678-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama, J., Y. Cao, T. Horii, S. Sakuda, A. D. Akkermans, W. M. de Vos, and H. Nagasawa. 2001. Gelatinase biosynthesis-activating pheromone: a peptide lactone that mediates a quorum sensing in Enterococcus faecalis. Mol. Microbiol. 41:145-154. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama, J., Y. Cao, T. Horii, S. Sakuda, and H. Nagasawa. 2001. Chemical synthesis and biological activity of the gelatinase biosynthesis-activating pheromone of Enterococcus faecalis and its analogs. Biosci. Biotechnol. Biochem. 65:2322-2325. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, J., R. Kariyama, and H. Kumon. 2002. Description of a 23.9-kilobase chromosomal deletion containing a region encoding fsr genes which mainly determines the gelatinase-negative phenotype of clinical isolates of Enterococcus faecalis in urine. Appl. Environ. Microbiol. 68:3152-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 34.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect. Immun. 68:2579-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J. Bacteriol. 183:3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice, L. B., L. Carias, S. Rudin, C. Vael, H. Goossens, C. Konstabel, I. Klare, S. R. Nallapareddy, W. Huang, and B. E. Murray. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508-512. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, J. C., K. V. Singh, P. C. Okhuysen, and B. E. Murray. 2004. Molecular epidemiology of the fsr locus and of gelatinase production among different subsets of Enterococcus faecalis isolates. J. Clin. Microbiol. 42:2317-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roof, D. M., and J. R. Roth. 1992. Autogenous regulation of ethanolamine utilization by a transcriptional activator of the eut operon in Salmonella typhimurium. J. Bacteriol. 174:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosinha, G. M., D. A. Freitas, A. Miyoshi, V. Azevedo, E. Campos, S. L. Cravero, O. Rossetti, G. Splitter, and S. C. Oliveira. 2002. Identification and characterization of a Brucella abortus ATP-binding cassette transporter homolog to Rhizobium meliloti ExsA and its role in virulence and protection in mice. Infect. Immun. 70:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Said-Salim, B., P. M. Dunman, F. M. McAleese, D. Macapagal, E. Murphy, P. J. McNamara, S. Arvidson, T. J. Foster, S. J. Projan, and B. N. Kreiswirth. 2003. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 185:610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapolsky, R. 2005. Presented at the 13th International Conference on Bacilli, San Diego, California.
- 42.Sheppard, D. E., J. T. Penrod, T. Bobik, E. Kofoid, and J. R. Roth. 2004. Evidence that a B12-adenosyl transferase is encoded within the ethanolamine operon of Salmonella enterica. J. Bacteriol. 186:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sifri, C. D., E. Mylonakis, K. V. Singh, X. Qin, D. A. Garsin, B. E. Murray, F. M. Ausubel, and S. B. Calderwood. 2002. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 70:5647-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, K. V., S. R. Nallapareddy, E. C. Nannini, and B. E. Murray. 2005. Fsr-independent production of protease(s) may explain the lack of attenuation of an Enterococcus faecalis fsr mutant versus a gelE-sprE mutant in induction of endocarditis. Infect. Immun. 73:4888-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturme, M. H., J. Nakayama, D. Molenaar, Y. Murakami, R. Kunugi, T. Fujii, E. E. Vaughan, M. Kleerebezem, and W. M. de Vos. 2005. An agr-like two-component regulatory system in Lactobacillus plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J. Bacteriol. 187:5224-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng, F., L. Wang, K. V. Singh, B. E. Murray, and G. M. Weinstock. 2002. Involvement of PhoP-PhoS homologs in Enterococcus faecalis virulence. Infect. Immun. 70:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verneuil, N., M. Sanguinetti, Y. Le Breton, B. Posteraro, G. Fadda, Y. Auffray, A. Hartke, and J. C. Giard. 2004. Effects of the Enterococcus faecalis hypR gene encoding a new transcriptional regulator on oxidative stress response and intracellular survival within macrophages. Infect. Immun. 72:4424-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waters, C. M., M. H. Antiporta, B. E. Murray, and G. M. Dunny. 2003. Role of the Enterococcus faecalis GelE protease in determination of cellular chain length, supernatant pheromone levels, and degradation of fibrin and misfolded surface proteins. J. Bacteriol. 185:3613-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Way, S. S., A. C. Borczuk, and M. B. Goldberg. 1999. Adaptive immune response to Shigella flexneri 2a cydC in immunocompetent mice and mice lacking immunoglobulin A. Infect. Immun. 67:2001-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Way, S. S., S. Sallustio, R. S. Magliozzo, and M. B. Goldberg. 1999. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, D. A. 1973. Phospholipid composition of mammalian tissues. Elsevier Scientific Publishing Company, New York, N.Y.
- 52.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, Y., J. M. Rivas, E. L. Brown, X. Liang, and M. Hook. 2004. Virulence potential of the staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J. Infect. Dis. 189:2323-2333. [DOI] [PubMed] [Google Scholar]
- 54.Zeng, J., F. Teng, and B. E. Murray. 2005. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect. Immun. 73:1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.