Abstract
Strains of Pseudomonas aeruginosa secrete one of three pyoverdine siderophores (types I to III). We have characterized a gene, pvdYII (for the pvdY gene present in type II P. aeruginosa strains), that is only present in strains that make type II pyoverdine. A mutation in pvdYII prevented pyoverdine synthesis. Bioinformatic, genetic, and biochemical approaches indicate that the PvdYII enzyme catalyzes acetylation of hydroxyornithine. Expression of pvdYII is repressed by the presence of iron and upregulated by the presence of type II pyoverdine. Characterization of pvdYII provides insights into the molecular basis for production of different pyoverdines by different strains of P. aeruginosa.
Pyoverdines are siderophores that are secreted by fluorescent pseudomonads and are efficient iron-scavenging compounds (6, 19). Over 50 pyoverdines are known, and all of these contain a dihydroxyquinoline-type chromophore; this is attached to a strain-specific peptide that contains unusual amino acids, such as D-isomers and amino acids that are not usually found in biomolecules, and an acyl group that varies depending on the growth conditions (Fig. 1). Ferri-pyoverdine complexes are recognized by receptor proteins located at the surfaces of the cells, and the iron is taken up by the bacteria in an energy-dependent process (reviewed in reference 27). Pyoverdines contribute to the ability of P. aeruginosa to cause infection (17, 32).
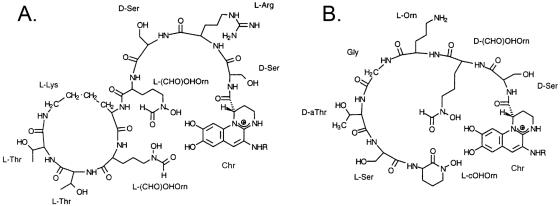
FIG. 1.
Type I and II pyoverdines. (A) Type I pyoverdine (5). (B) Type II pyoverdine (33). Chr, dihydroxyquinoline-type chromophoric group; L-(CHO)OHOrn, δN-formyl-δN-hydroxyornithine; L-cOHOrn, N-hydroxy-cyclo-ornithine; R, succinyl, succinamide, or α-ketoglutaryl residue. Figure adapted from reference 18, with permission.
Strains of P. aeruginosa secrete one of three pyoverdines (types I to III) (18). Genes and enzymes required for pyoverdine synthesis in strain PAO, which secretes type I pyoverdine, have been characterized experimentally (1, 4, 12, 15, 16, 21, 23, 31, 34, 35). This has revealed a biosynthetic pathway in which a pyoverdine precursor is assembled by nonribosomal peptide synthetases (NRPSs), with other enzymes providing the unusual amino acid substrates for the NRPSs and modifying the precursor peptide to yield the mature pyoverdine. Genomic analyses imply that the pathway of synthesis is similar in outline in other Pseudomonas species (25), and this is supported by experimental evidence (2, 21, 24). Pyoverdine synthesis has not been studied experimentally in strains of P. aeruginosa other than PAO1. However, recent genomic analysis shows that strains that make different pyoverdines share many pyoverdine synthesis genes with strain PAO, but they contain additional genes that are not present in strain PAO and are proposed to be required for pyoverdine synthesis (29).
In strain PAO, the pvdS gene is adjacent to a gene, pvdY, that is of unknown function, although a mutation in pvdY resulted in reduced pyoverdine synthesis (23). pvdY is also adjacent to pvdX (Fig. 2), a gene of unknown function. The same arrangement of genes has been demonstrated in strains of P. aeruginosa that make other pyoverdines (29). In strains that make type II pyoverdine, the pvdY gene (unlike the pvdS and pvdX genes) has very little sequence similarity to the pvdY gene in strains that make type I or type III pyoverdines, and it is not alignable by pairwise alignment (29) (L. W. Martin, M. Wallace, and I. L. Lamont, unpublished data).
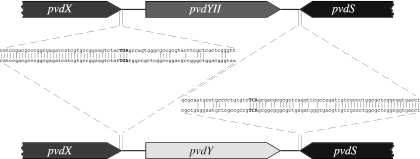
FIG. 2.
Organization of pvdXYS genes. The organization of the pvdX, pvdY, and pvdS genes from P. aeruginosa strain Pa4 (top) and strain PAO (bottom) is shown. The genes are not drawn to scale; the pvdY protein-coding sequence is 468 bp, and the pvdYII protein-coding sequence is 912 bp. The stop codons for pvdX and pbdS are in bold.
The pvdY gene present in type II strains is referred to here as pvdYII. The aim of this research was to investigate the function of pvdYII in P. aeruginosa strain Pa4 that makes type II pyoverdine (18). We first amplified a DNA fragment carrying the gene from genomic DNA from P. aeruginosa strain Pa4 by PCR using suitable primers (5′-CCCTCTAGACAAGGAACTGGGCGTCTCG-3′ and 5′GGGAAGCTTCTGAACTGCATCCACCACCTG-3′, with introduced XbaI and HindIII restriction sites shown in boldface). This gave rise to a product of about 1.5 kb that was cloned into pGEM-T Easy (Promega) using the manufacturer's protocol. DNA sequencing (Allan Wilson Centre Genome Service, Palmerston North, New Zealand) and analysis revealed the presence of the pvdYII gene flanked by the pvdS and pvdX genes (Fig. 2), as is found in other strains that produce type II pyoverdine. Alignment of the pvdS and pvdX gene sequences shows a very high (>99%) degree of nucleotide sequence identity (Fig. 2 and data not shown), as has been found for other genes that are present in multiple strains of P. aeruginosa (10, 30). The amount of sequence similarity changes abruptly (at the end of pvdX) or slightly more gradually (towards the end of pvdS) (Fig. 2) so that there is no significant sequence similarity in the intergenic regions or between the pvdY genes. The genetic mechanisms that led to pvdY replacing (or being replaced by) pvdYII during the evolution of P. aeruginosa genomes are not clear.
The similarity of pvdYII to siderophore synthesis genes (see below) and its linkage to pvdS raised the possibility that pvdYII is required for pyoverdine synthesis by Pa4. This hypothesis was tested by engineering a Pa4 mutant strain in which the wild-type allele was replaced by a mutant allele. The XbaI-HindIII restriction fragment containing pvdYII was excised from the pGEM-T Easy clone and subcloned into pEX18Gm (8) using standard methods (26). A promoterless lacZ gene cassette was excised from pZ1918 (28) using SphI and was cloned into the unique SphI site in pvdYII in the pEXGm plasmid; two alleles were obtained, with lacZ in the sense (pvdYII::lacZ) or antisense (pvdYII::Zcal) orientation. Wild-type pvdYII in strain Pa4 was then replaced with the mutant alleles using methods described elsewhere (8). Replacement of the wild-type gene by the mutant alleles was confirmed by Southern blotting (data not shown).
The resulting pvdYII mutants failed to make detectable pyoverdine and were unable to grow in the presence of the iron-chelating compound ethylenediamine(o-hydroxy)phenylacetic acid (EDDHA) that inhibits growth of Pvd− mutants (12) (Table 1), showing that pvdYII is indeed required for pyoverdine synthesis by Pa4. The wild-type pvdYII gene was subcloned as a HindIII-XbaI fragment from pGEM-T Easy into the mini-CTX2 vector and then integrated into the chromosome of the Pa4 pvdYII::lacZ mutant strain as described previously (9). This restored the ability of the bacteria to make pyoverdine and to grow on medium containing EDDHA (Table 1), confirming that the inability of the mutant to synthesize pyoverdine was due to the absence of functional pvdYII.
TABLE 1.
Synthesis of pyoverdines by strains of P. aeruginosa used in this study
| Strain | Pyoverdine synthesisa | Growth with EDDHAb |
|---|---|---|
| PAO | ++ | + |
| PAO pvdF | − | − |
| PAO pvdF(ctx::pvdF) | ++ | + |
| PAO pvdF(ctx::pvdYII) | ++ | + |
| Pa4 | + | + |
| Pa4 pvdYII::lacZ | − | − |
| Pa4 pvdYII::Zcal | − | NDc |
| Pa4 pvdYII(ctx::pvdF) | − | − |
| Pa4 pvdYII(ctx::pvdYII) | + | + |
Pyoverdine synthesis (as indicated by production of a fluorescent yellow-green pigment on Kings B agar and by the presence of a characteristic absorbance spectrum in Kings B broth) was detected as described previously (12, 15). ++, large amounts of pyoverdine were synthesized; +, significant amounts of pyoverdine were synthesized; −, no detectable pyoverdine was synthesized.
Bacterial growth was recorded after growth at 37°C on Kings B agar containing the iron-chelating compound ethylenediamine(o-hydroxy)phenylacetic acid (EDDHA) (200 μg ml−1). +, good growth after 24 h of incubation; −, no detectable growth after 24 h of incubation.
ND, not determined.
Expression of pyoverdine synthesis genes in P. aeruginosa strain PAO is dependent on the alternative sigma factor PvdS (13, 20), and it is likely that the same is true in other strains of P. aeruginosa. The pvdYII open reading frame in strain Pa4 is preceded by a DNA sequence (TAAAT-N16-CGT) that is present in PvdS-dependent promoters (23, 36, 37), so that it is very likely that expression of pvdYII is dependent at least in part on PvdS. The Pa4 pvdYII mutant contains a pvdYII::lacZ reporter gene fusion, and this was used to investigate gene expression using the same methods as those described previously (11, 37). In three independent experiments, bacteria grown in Kings B broth gave 3,137 U of β-galactosidase (standard deviation [SD], 306 U). Assays with the Pa4 pvdYII::Zcal strain, in which the lacZ reporter gene is in the antisense orientation to pvdYII, gave 29 U of β-galactosidase, consistent with lacZ expression in the Pa4 pvdYII::lacZ strain being dependent on the pvdYII promoter. Growth of the Pa4 pvdYII::lacZ strain in Kings B broth containing FeCl3 (60 μg ml−1) gave 568 U (SD, 88 U), showing that iron results in repression of pvdYII gene expression in strain Pa4. This reflects pvd gene expression in strain PAO which is strongly down-regulated in iron-replete cells due to the Fur protein that represses expression of pvdS (22).
In P. aeruginosa strain PAO, PvdS-dependent gene expression is lower in Pvd− mutants than in wild-type bacteria and can be increased to wild-type levels by the addition of pyoverdine (11). This is due to a pyoverdine-responsive transmembrane regulatory system that controls the activity of PvdS. The addition of type II pyoverdine to Pa4 pvdYII::lacZ bacteria growing in Kings B broth resulted in 5,861 U of β-galactosidase (SD, 1,001 U), an increase in pvdYII::lacZ gene expression relative to bacteria without added pyoverdine. This is consistent with the existence of a pyoverdine-inducible signaling pathway, although the increase in gene expression was less than twofold. This is less than that seen with the pvdE gene in strain PAO, where the increase in expression is about fivefold (11), or with the psbA pyoverdine biosynthesis gene in P. fluorescens strain B10 (3), although it should be noted that expression of the pvdA gene in P. aeruginosa PAO1 did not show increased expression when pyoverdine was added (3).
The predicted product of pvdYII is a 39-kDa protein. BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/) showed that this protein has highest sequence similarities with acetyl transferases that are involved in siderophore synthesis. These include IucB that is required for aerobactin synthesis in Escherichia coli (7) (41% amino acid identity), RhbD, a putative acetyltransferase involved in rhizobactin synthesis in Sinorhizobium meliloti (14) (52% amino acid identity), and an acetylase that is involved in siderophore synthesis in P. fluorescens (21) (69% amino acid identity). IucB and the P. fluorescens enzyme have been shown to catalyze acetylation of hydroxylamine groups to form iron-binding hydroxamate end groups. The pyoverdine synthesized by strain Pa4 does not contain acetyl hydroxamate groups but contains a cyclic hydroxamate (Fig. 1). We hypothesized that pvdYII catalyzes acetylation of N-hydroxyornithine as part of the process of synthesis of this hydroxamate.
Type I pyoverdine contains two formyl hydroxamate groups, and synthesis of these is catalyzed by the PvdF enzyme, which formylates a hydroxyornithine precursor (15). As PvdF and pvdYII may catalyze synthesis of different hydroxamate groups (formyl- or acetyl-hydroxamate, respectively), we tested the possibility that pvdF from strain PAO could substitute for pvdYII from strain Pa4 and vice versa. A 1.5-kb DNA fragment containing the pvdF gene from P. aeruginosa PAO was amplified by PCR using primers flanking the gene (5′-GCGTCTAGATCATAGTTGCTTCCCGGA, with an introduced XbaI site shown in boldface, and 5′-ACGGCAACGTCTACGAG-3′), cloned into pGEM-T Easy, and then subcloned into the mini-CTX2 vector. This construct was then integrated into the chromosomes of PAO pvdF and Pa4 pvdYII mutant strains, and the mini-ctx::pvdYII construct was integrated into the chromosome of the PAO pvdF mutant. The resulting strains were tested for the ability to synthesize pyoverdine (Table 1). The ctx::pvdYII construct restored the ability of the PAO pvdF mutant to make pyoverdine, but the ctx::pvdF construct did not enable synthesis of pyoverdine by the Pa4 pvdYII mutant.
Pyoverdine produced by the PAO pvdF(ctx::pvdYII) strain, as well as that made by PAO pvdF(ctx::pvdF) and Pa4 pvdYII(ctx::pvdYII), was purified as described previously (18). The pyoverdines were analyzed at the Protein Microchemistry Facility of the University of Otago by matrix-assisted laser desorption ionization-time of flight mass spectrometry (average of at least five runs) using a Thermo Finnigan Lasermat 2000 in conjunction with an alpha-cyano-4-hydroxycinnamic acid matrix and the internal calibrants Bradykinin (1,060.2 Da) and Renin substrate (1,759.0 Da); in addition, for the pyoverdine produced by the PAO pvdF(ctx::pvdYII) strain, electrospray mass spectrometry was performed using a Thermo Finnigan LCQ Deca. The pyoverdine produced by PAO pvdF(ctx::pvdF) was 1,336 Da, and that produced by Pa4 pvdYII(ctx::pvdYII) was 1,094 Da. These are the same as type I and II pyoverdines (5, 33), showing that complementation of the genes resulted in synthesis of wild-type pyoverdines. The PAO pvdF(ctx::pvdYII) pyoverdine had a mass of 1,364 Da by mass spectrometry. This is consistent with a form of type I pyoverdine in which the formyl pvdYII residues have been replaced by acetyl hydroxyornithine, which would increase the Mr by 28. This pyoverdine was also analyzed by electrospray mass spectrometry, which gave an Mr of 1,362.5.
Collectively, these data provide strong evidence that pvdYII is required for synthesis of type II pyoverdine by P. aeruginosa strain Pa4 and that it catalyzes acetylation of hydroxyornithine. The pvdYII gene is restricted to strains of P. aeruginosa that make type II pyoverdine (29), and its characterization advances our understanding of the molecular basis for production of different pyoverdines by different strains of P. aeruginosa. Type II pyoverdine does not contain acetyl hydroxyornithine but does contain a terminal cyclized hydroxyornithine (Fig. 1). Our current model is that acetyl hydroxyornithine is incorporated into a peptide precursor of type II pyoverdine by the relevant NRPS, with the acetyl group being removed during release of the peptide from the NRPS and concomitant cyclization of the hydroxyornithine. Alternatively, the substrate of the NRPS may be ornithine itself, with pvdYII catalyzing its acetylation following incorporation into the precursor peptide but prior to the release of the peptide from the NRPS. In either case, absence of acetyl hydroxyornithine is likely to preclude the cyclization of the terminal hydroxyornithine derivative and release of the precursor peptide from the NRPS. A requirement for acetyl hydroxyornithine prior to cyclization of the ornithine residue would explain the observation that pvdYII could complement a pvdF mutation in P. aeruginosa PAO but pvdF could not complement a pvdYII mutation.
Nucleotide sequence accession numbers.
The Pa4 pvdYII sequence reported here has been assigned the GenBank accession number DQ328792.
Acknowledgments
We are very grateful to Jean-Marie Meyer for providing strain Pa4, to Herbert Schweizer for providing plasmids, and to Paul Beare for his assistance with purification of pyoverdine.
REFERENCES
- 1.Ackerley, D. F., T. T. Caradoc-Davies, and I. L. Lamont. 2003. Substrate specificity of the nonribosomal peptide synthetase PvdD from Pseudomonas aeruginosa. J. Bacteriol. 185:2848-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosi, C., L. Leoni, L. Putignani, N. Orsi, and P. Visca. 2000. Pseudobactin biogenesis in the plant growth-promoting rhizobacterium Pseudomonas strain B10: identification and functional analysis of the l-ornithine N5-oxygenase (psbA) gene. J. Bacteriol. 182:6233-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosi, C., L. Leoni, and P. Visca. 2002. Different responses of pyoverdine genes to autoinduction in Pseudomonas aeruginosa and the group Pseudomonas fluorescens-Pseudomonas putida. Appl. Environ. Microbiol. 68:4122-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baysse, C., H. Budzikiewicz, D. Uria Fernandez, and P. Cornelis. 2002. Impaired maturation of the siderophore pyoverdine chromophore in Pseudomonas fluorescens ATCC 17400 deficient for the cytochrome c biogenesis protein CcmC. FEBS Lett. 523:23-28. [DOI] [PubMed] [Google Scholar]
- 5.Briskot, G., K. Taraz, and H. Budzikiewicz. 1989. Pyoverdin-type siderophores from Pseudomonas aeruginosa. Liebigs Ann. Chem 1989:375-384. [Google Scholar]
- 6.Budzikiewicz, H. 2004. Siderophores of the Pseudomonadaceae sensu stricto (fluorescent and non-fluorescent Pseudomonas spp.). Fortschr. Chem. Org. Naturst. 87:81-237. [DOI] [PubMed] [Google Scholar]
- 7.de Lorenzo, V., A. Bindereif, B. H. Paw, and J. B. Neilands. 1986. Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J. Bacteriol. 165:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 9.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 10.Kiewitz, C., and B. Tummler. 2000. Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J. Bacteriol. 182:3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamont, I. L., and L. W. Martin. 2003. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiology 149:833-842. [DOI] [PubMed] [Google Scholar]
- 13.Leoni, L., N. Orsi, V. d. Lorenzo, and P. Visca. 2000. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J. Bacteriol. 182:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch, D., J. O'Brien, T. Welch, P. Clarke, P. O. Cuiv, J. H. Crosa, and M. O'Connell. 2001. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 183:2576-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMorran, B. J., H. M. C. S. Kumara, K. Sullivan, and I. L. Lamont. 2001. Involvement of a transformylase enzyme in siderophore synthesis in Pseudomonas aeruginosa. Microbiology 147:1517-1524. [DOI] [PubMed] [Google Scholar]
- 16.McMorran, B. J., M. E. Merriman, I. T. Rombel, and I. L. Lamont. 1996. Characterisation of the pvdE gene which is required for pyoverdine synthesis in Pseudomonas aeruginosa. Gene 176:55-59. [DOI] [PubMed] [Google Scholar]
- 17.Meyer, J.-M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdine is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer, J.-M., A. Stintzi, D. D. Vos, P. Cornelis, R. Tappe, K. Taraz, and H. Budzikiewicz. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35-43. [DOI] [PubMed] [Google Scholar]
- 19.Meyer, J. M. 2000. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol. 174:135-142. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki, H., H. Kato, T. Nakazawa, and M. Tsuda. 1995. A positive regulatory gene, pvdS, for expression of pyoverdine biosynthetic genes in Pseudomonas aeruginosa PAO. Mol. Gen. Genet. 248:17-24. [DOI] [PubMed] [Google Scholar]
- 21.Mossialos, D., U. Ochsner, C. Baysse, P. Chablain, J. P. Pirnay, N. Koedam, H. Budzikiewicz, D. U. Fernandez, M. Schafer, J. Ravel, and P. Cornelis. 2002. Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol. Microbiol. 45:1673-1685. [DOI] [PubMed] [Google Scholar]
- 22.Ochsner, U. A., A. I. Vasil, and M. L. Vasil. 1995. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J. Bacteriol. 177:7194-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 24.Putignani, L., C. Ambrosi, P. Ascenzi, and P. Visca. 2004. Expression of L-ornithine Nδ-oxygenase (PvdA) in fluorescent Pseudomonas species: an immunochemical and in silico study. Biochem. Biophys. Res. Commun. 313:245-257. [DOI] [PubMed] [Google Scholar]
- 25.Ravel, J., and P. Cornelis. 2003. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 11:195-200. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., D. W. Russell, and N. Irwin. 2000. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Schalk, I. J., W. W. Yue, and S. K. Buchanan. 2004. Recognition of iron-free siderophores by TonB-dependent iron transporters. Mol. Microbiol. 54:14-22. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer, H. P. 1993. Two plasmids, X1918 and Z1918, for easy recovery of they xylE and lacZ reporter genes. Gene 134:89-91. [DOI] [PubMed] [Google Scholar]
- 29.Smith, E. E., E. H. Sims, D. H. Spencer, R. Kaul, and M. V. Olson. 2005. Evidence for diversifying selection at the pyoverdine locus of Pseudomonas aeruginosa. J. Bacteriol. 187:2138-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer, D. H., A. Kas, E. E. Smith, C. K. Raymond, E. H. Sims, M. Hastings, J. L. Burns, R. Kaul, and M. V. Olson. 2003. Whole-genome sequence variation among multiple isolates of Pseudomonas aeruginosa. J. Bacteriol. 185:1316-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stintzi, A., Z. Johnson, M. Stonehouse, U. Ochsner, J.-M. Meyer, M. L. Vasil, and K. Poole. 1999. The pvc gene cluster of Pseudomonas aeruginosa: role in synthesis of the pyoverdine chromophore and regulation by PtxR and PvdS. J. Bacteriol. 181:4118-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunocompromised mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tappe, R., K. Taraz, H. Budzikiewicz, J.-M. Meyer, and L. F. Lefevre. 1993. Structure elucidation of a pyoverdine produced by Pseudomonas aeruginosa ATCC 27853. J. Prakt. Chem. 335:83-87. [Google Scholar]
- 34.Vandenende, C. S., M. Vlasschaert, and S. Y. Seah. 2004. Functional characterization of an aminotransferase required for pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:5596-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visca, P., A. Ciervo, and N. Orsi. 1994. Cloning and nucleotide sequence of the pvdA gene encoding the pyoverdine biosynthetic enzyme L-ornithine N5-oxygenase in Pseudomonas aeruginosa. J. Bacteriol. 176:1128-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, M. J., B. J. McMorran, and I. L. Lamont. 2001. Analysis of promoters recognized by PvdS, an extra-cytoplasmic function sigma factor protein from Pseudomonas aeruginosa. J. Bacteriol. 183:2151-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]