Abstract
Desulfovibrio gigas flavodiiron protein (FDP), rubredoxin:oxygen oxidoreductase (ROO), was proposed to be the terminal oxidase of a soluble electron transfer chain coupling NADH oxidation to oxygen reduction. However, several members from the FDP family, to which ROO belongs, revealed nitric oxide (NO) reductase activity. Therefore, the protection afforded by ROO against the cytotoxic effects of NO was here investigated. The NO and oxygen reductase activities of recombinant ROO in vitro were tested by amperometric methods, and the enzyme was shown to effectively reduce NO and O2. Functional complementation studies of an Escherichia coli mutant strain lacking the ROO homologue flavorubredoxin, an NO reductase, showed that ROO restores the anaerobic growth phenotype of cultures exposed to otherwise-toxic levels of exogenous NO. Additional studies in vivo using a D. gigas roo-deleted strain confirmed an increased sensitivity to NO of the mutant strain in comparison to the wild type. This effect is more pronounced when using the nitrosating agent S-nitrosoglutathione (GSNO), which effectively impairs the growth of the D. gigas Δroo strain. roo is constitutively expressed in D. gigas under all conditions tested. However, real-time reverse transcription-PCR analysis revealed a twofold induction of mRNA levels upon exposure to GSNO, suggesting regulation at the transcription level by NO. The newly proposed role of D. gigas ROO as an NO reductase combined with the O2 reductase activity reveals a versatility which appears to afford protection to D. gigas at the onset of both oxidative and nitrosative stresses.
Desulfovibrio gigas is a sulfate-reducing bacterium considered to be a strict anaerobe. However, there is growing evidence that this organism can survive and probably even profit energetically from transient exposure to oxygen (8, 23). Like other isolates from the Desulfovibrio genus, D. gigas has metal-binding enzymes for the detoxification of molecular oxygen (rubredoxin:oxygen oxidoreductase [ROO] and cytochrome bd) (8, 23), its reactive species (catalase and superoxide dismutase) (9), and superoxide reductase (34). The first proposed D. gigas defense mechanism against O2 involved a flavometalloprotein, ROO (8), which accomplishes full oxygen reduction to water by accepting electrons from reduced rubredoxin (Rd). Rd is in turn reduced by NADH:rubredoxin oxidoreductase (7, 8).
ROO belongs to the flavodiiron protein (FDP) family, whose members are widespread in strict and facultative anaerobic Archaea and Bacteria members, as well as in anaerobic protozoa (1, 29, 30). The resolution of its crystallographic structure (12) revealed that each monomer of this homodimeric protein consists of two structural modules, a C-terminal flavin mononucleotide-binding flavodoxin-like domain and an N-terminal metallo-β-lactamase-like domain. More recently, the structure of the FDP from Moorella thermoacetica was also solved (33).
Although a role in oxygen reduction was initially assigned to the FDP family, recent reports contributed to the establishment of members of this protein family as NO reductases. The first evidence of NO reduction activity concerned Escherichia coli flavorubredoxin (FlRd). A role in anaerobic NO reduction was proposed for FlRd on the basis of an increased sensitivity to NO of an E. coli norV (the gene encoding FlRd) mutant (13), which was further confirmed by the NO reductase activity of the enzyme in vitro (15) and by transcriptional analysis of E. coli grown under nitrosative stress, both aerobically (25) and anaerobically (18). Subsequently, the same NO reductase activity was determined for the FDPs from M. thermoacetica (32) and Desulfovibrio vulgaris (33).
The presence of protective mechanisms against NO in Desulfovibrio species (besides D. gigas and D. vulgaris, other Desulfovibrio species have FDP orthologues present in their genomes) raises the possibility that these organisms may face nitrosative stress in their living environments. They may indeed have to cope with NO formed and released in the process of nitrite reduction by coexisting denitrifying organisms. On the other hand, as some of these organisms inhabit the mammalian digestive tract, associated with chronic inflammatory diseases (14, 24), they may locally be subjected to the immune response mechanisms of their hosts at the onset of infection and inflammatory processes, including NO production and release by activated macrophages. The existence of FDPs in Desulfovibrio species thus appears to constitute another stress resistance strategy of these versatile organisms.
The present work aims to investigate the role of ROO in the protection of D. gigas against NO, using a combination of approaches in vitro and in vivo. The NO and O2 reductase activities of recombinant ROO in vitro were measured by amperometric methods, and the approach in vivo involved complementation studies performed with an E. coli norV mutant strain, as well as construction and phenotypic analysis of a D. gigas roo mutant strain. The results are discussed in the light of the versatility of ROO to act either as an NO or an oxygen reductase.
MATERIALS AND METHODS
Cloning, overexpression, and purification of recombinant ROO.
ROO was cloned in the expression vector pET-30a (Novagene), using restriction sites for NdeI and XhoI. The clone obtained was confirmed by sequencing, performed with the Thermo Sequenase Cycle Sequencing kit (Amersham) and with the ABI Prism 373A Automatic Sequencer using the ABI Prism DyeDeoxy Terminator Cycle Sequencing kit. Recombinant expression of ROO was performed with E. coli strain BL21(DE3) (Novagene), using M9 minimal medium (2) with kanamycin (30 mg/ml) supplemented with FeSO4 (10 μM) at 30°C. Isopropyl-beta-d-thiogalactopyranoside (IPTG; 1 mM) was used as an inducer at an optical density at 600 nm (OD600) of ∼0.6, and cultures were allowed to grow for 7 h before harvest of the cells by centrifugation. E. coli cells overexpressing ROO were disrupted in two cycles in a French press minicell at 1,000 lb/in2. The soluble extract was separated from the membrane fraction by ultracentrifugation for 2 h at 100,000 × g and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), using molecular mass markers (Fermentas). The obtained soluble extract was dialyzed overnight at 4°C against 5 mM Tris-HCl, pH 7.6, with 9% glycerol (buffer A) and then loaded onto a 60 ml Q-Sepharose Fast Flow column (Amersham Pharmacia) previously equilibrated with 20 mM Tris-HCl and 18% glycerol, pH 7.5 (buffer B). An NaCl gradient of 8 column volumes (from buffer B to buffer C, i.e., buffer B with 1 M NaCl) was applied at a flow rate of 2.5 ml/min, and ROO was eluted at approximately 350 mM NaCl, as indicated by the mild yellow color, and confirmed by UV-visible spectroscopy. The pooled fractions were then dialyzed overnight against buffer A and subsequently applied to a 50-ml Fractogel TMAE column (Merck), previously equilibrated with buffer B. ROO eluted at approximately 250 mM NaCl, at a flow rate of 1.5 ml/min, using an 8-column-volume gradient of buffer B to buffer C. This step yielded pure ROO, as judged by SDS-PAGE.
Biochemical analysis of recombinant ROO.
The purity of recombinant ROO was assessed by 12% SDS-PAGE (20). ROO flavin cofactor analysis was performed as described previously (38), and its iron content was determined by the 2,4,6-tripyridyl 1,3,5-triazine method (11). The amount of total protein was quantified by the bicinchoninic acid assay method (36).
Amperometric monitoring of nitric oxide and oxygen reduction.
NO consumption measurements were carried out using a World Precision Instruments ISO-NOP 2-mm electrode at room temperature as described previously (15). Reduced D. gigas rubredoxin was prepared separately by anaerobically incubating 415 μM Rd with 48 nM E. coli FlRd-reductase and 1 mM NADH. D. gigas rubredoxin and E. coli flavorubredoxin reductase were overexpressed in E. coli and purified as described previously (15, 21).
Oxygen consumption rates were measured with a Clark-type O2 electrode plugged into a Biological Oxygen monitor (model 5300; Yellow Springs). Rubredoxin was reduced in situ with 400 nM E. coli FlRd reductase and 1 mM NADH prior to the addition of ROO to the reaction mixture. Catalase (Sigma) was added to the reaction mixture to test the production of hydrogen peroxide upon oxygen consumption. The experiments were carried out at 25°C in an air-equilibrated buffer (50 mM Tris-HCl, pH 7.5, containing 18% glycerol), i.e., with approximately 240 μM O2. Since full reduction of oxygen to water requires four electrons, ROO will oxidize four Rd (one-electron carrier) molecules to catalyze this reaction to completeness. To check whether the O2 or NO reductase activities were limited by electron donation from reduced Rd, the concentration of the latter was varied, and the O2 and NO consumption rates were measured.
Functional complementation studies.
roo was cloned into the expression vector pFLAG-CTC (Sigma), using restriction sites BamHI and XhoI, and the resulting plasmid construction pFLAG-CTC(ROO) was confirmed by sequencing, as described above. Anaerobic cultures of E. coli AG300 (containing the norV gene disrupted) transformed with either pFLAG-CTC (Sigma) or pFLAG-CTC(ROO) were grown without shaking at 37°C under N2 gas in medium prepared as described previously (32) with ampicillin (100 μg/ml).
roo expression was induced by addition of IPTG (1 mM) to 80-ml anaerobic cultures of E. coli AG300[pFLAG-CTC] and AG300[pFLAG-CTC(ROO)], at OD550 of ∼0.3. Where required, 50 μM NO or 1 mM S-nitrosoglutathione (GSNO) was added to the cultures 1 h after induction. Culture growth was followed by measurement of OD at 550 nm. NO-saturated solutions were prepared as described previously (3) and used immediately afterward. Residual oxygen was removed from the solutions stored in rubber seal-capped flasks by bubbling with oxygen-free argon prior to saturation with NO. NO was depleted from contaminants such as higher N oxides (mostly NO2) by being passed first through 5 M NaOH in a gas scrubbing bottle and then through a second bottle containing water, thus avoiding aerosol contamination. GSNO stock solutions were prepared as described previously (37) by mixing S-glutathiol (Sigma) with sodium nitrite under acidic conditions.
D. gigas growth and roo deletion.
D. gigas cells were grown anaerobically at 37°C in lactate-sulfate medium (22).
To determine the function of ROO in vivo, a null mutant for this gene in D. gigas was generated by gene replacement with a kanamycin resistance gene by homologous recombination (5, 27).
Plasmid construction.
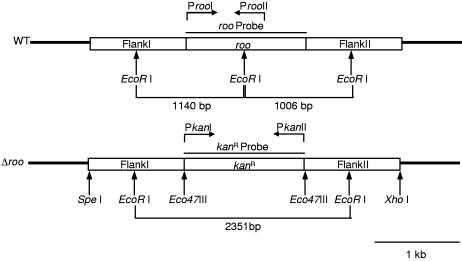
The flanking regions of the gene encoding ROO were amplified by PCR, including appropriate restriction sites for cloning in the vector pZErO-1 (Invitrogen), for ligation with the amplified kanamycin resistance gene. The kanamycin resistance gene from pJRD215 was amplified by PCR, using primers with appropriate restriction sites for ligation with the cloned flanking regions of roo (see Fig. 5) The resulting plasmid used for D. gigas transformation consisted of the kanamycin resistance gene with the flanking regions of roo.
FIG. 5.
Restriction map of wild-type D. gigas and Δroo D. gigas. The primers used are indicated.
D. gigas transformation.
D. gigas cells to be transformed were prepared based on the method previously described (5, 27) from 500 ml of an early-stationary-phase culture (OD600, ∼0.7 to 0.8). Transformation was performed with 6 μg of the plasmid construct in 0.1-cm cuvettes by electroporation in a Bio-Rad Gene Pulser apparatus, setting the resistance to ∞, using a 0.7-kV voltage and a 3-μF capacitance. Immediately after electroporation, cells were inoculated in medium (22) and allowed to grow at 37°C. After a 5-h growth period, kanamycin was added to the medium (50 μg/ml), and the cultures were allowed to grow overnight. Cells were then subcultured three consecutive times in medium with kanamycin (50 μg/ml). Colonies were isolated in medium supplemented with agar (15 g/liter) and kanamycin (50 μg/ml) by the roll tube technique (5, 28). roo gene deletion was confirmed by PCR and Southern blot analyses.
Phenotypic analysis of the D. gigas Δroo strain.
Cells were grown anaerobically at 37°C in lactate-sulfate medium (22) until early log phase (OD600, ∼0.3 to 0.4). Where appropriate, 10 μM NO or 10 μM GSNO was added to the cultures. Growth of the cultures was monitored by OD600 nm.
D. gigas growth for mRNA expression analysis.
Cells were grown anaerobically as described above until early log phase. Where appropriate, exposure to NO, GSNO, or O2 was achieved by the addition of NO-saturated water to the cultures to a concentration of 150 μM NO, of GSNO solution to a concentration of 1 mM, or air-saturated water to a concentration of 60 μM O2 for 1 h. Exposure to 60 μM O2 was chosen according to the oxygen concentration at which Desulfovibrio desulfuricans CSN (DSM 9104), a close relative of D. gigas, was shown to survive (31) and also because this value is within the range of oxygen concentrations previously shown to induce expression of an ROO orthologue in Clostridium acetobutylicum (19).
RNA isolation and real-time reverse transcription-PCR (RT-PCR) analysis.
Total RNA was extracted as previously described (26) from early-log-phase D. gigas cells, grown as previously described.
Total RNA was treated with DNaseI RNase-free (Roche), and Transcriptor Reverse Transcriptase (Roche) was used to synthesize cDNA with the random primer p(dN)6, according to the manufacturer's instructions. To rule out the presence of DNA in the RNA preparation, a control reaction was performed without the RT (no-RT control). Amplification and quantification of cDNA was performed with a Light Cycler (Roche) with the DNA Master SYBR Green I kit (Roche), according to the manufacturer's instructions, using specific primers amplifying a region of 331 bp in roo. The housekeeping gene for 16S rRNA was used as the internal control. Relative standard curves were generated with duplicate serial dilutions of cDNA. The samples for comparison were analyzed as unknowns using duplicate dilutions of cDNA. The relative expression of roo (roo/16S) in the different conditions tested was normalized against its expression in cells grown in standard conditions.
RESULTS
Biochemical and spectroscopic analyses of recombinant ROO.
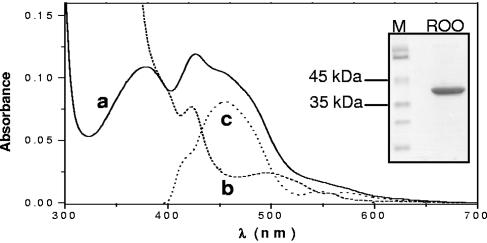
ROO was successfully overexpressed in E. coli and purified to homogeneity, as revealed by the SDS-PAGE analysis which displayed a single band of about 43 kDa (Fig. 1, inset), as predicted. Recombinant ROO was isolated with ∼2 irons and ∼0.8 flavin mononucleotides per monomer, as expected.
FIG. 1.
Biochemical and spectroscopic analyses of recombinant D. gigas ROO. Visible spectra of pure ROO recorded in different redox states. Line a (full), spectrum of an isolated ROO; line b (dashed), spectrum of reduced ROO (the sample from line a reduced with sodium dithionite); line c (dotted), redox spectrum obtained by subtracting line a from line b. Inset, SDS-PAGE analysis of purified recombinant D. gigas ROO.
The spectrum of recombinant oxidized D. gigas ROO (Fig. 1) was dominated by the flavin moiety, with two major bands around 380 nm and 450 nm. The band at 424 nm appeared to be due to a substoichiometric b-type heme. The low-absorbance band at approximately 500 nm observed in the spectrum of the reduced enzyme (Fig. 1, line b) remains to be assigned. The dominance of the flavin moiety was confirmed by subtracting the reduced ROO spectrum to the oxidized one (Fig. 1, line a). The redox spectrum thus obtained (Fig. 1, line c) displayed the features of the oxidized flavin moiety, with a maximum at 454 nm and the α and β bands at 548 and 571 nm, characteristic of reduced heme moieties. From the redox spectrum, it was also possible to estimate a heme content of about 0.02 to 0.04 mol heme/mol ROO. It should be noted that wild-type (wt) ROO also contains substoichiometric amounts of heme (16). The significance, if any, of the heme copurification, also observed for the D. vulgaris enzyme (33), is not known. Nevertheless, the very small amount of heme (<0.005 heme per protein molecule) present in the purified recombinant protein precludes any significant contribution to the NO reaction.
Oxygen consumption by recombinant D. gigas ROO.
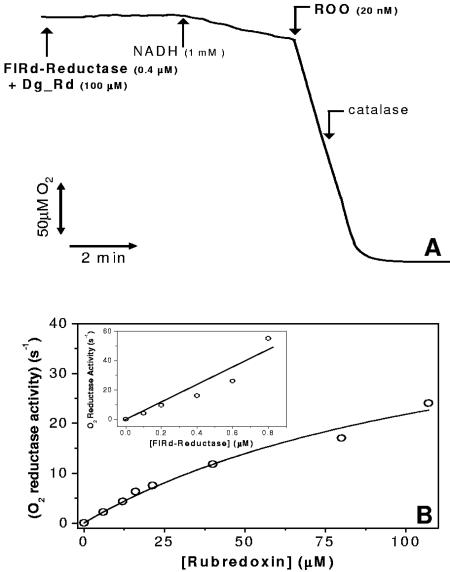
The oxygen reductase activity of recombinant D. gigas ROO was measured using reduced recombinant D. gigas rubredoxin as the electron donor (Fig. 2A). In the range of Rd concentrations tested, the oxygen reduction activity of ROO was shown to depend hyperbolically on the concentration of Rd (Fig. B). From the data, a maximal oxygen reductase activity can be estimated as 50.5 ± 10 μM O2 · s−1 · μM ROO−1. However, the observation that a large fraction of the Rd was immediately oxidized upon addition of ROO and remained so in the course of the assays suggested that rereduction of Rd by FlRd reductase could limit the overall reaction. This was further investigated by performing a set of experiments where the Rd concentration was kept constant and the FlRd reductase concentration was varied. Figure 2B, inset, displays the linear increase of the O2 reductase activity of ROO with increasing FlRd reductase concentrations, up to 1 μM. The feasibility of these assays at higher FlRd reductase concentrations was impaired by the O2 reductase activity of the FlRd reductase (17). The slow O2 consumption observed upon addition of FlRd reductase (Fig. 2A) became steeper with the increasing FlRd reductase concentration, enough to compromise the correct assignment of the O2 reductase activity to ROO. With these observations in mind, we envisage that the maximal oxygen reductase activity of recombinant ROO may be significantly higher than the rates reported here, i.e., when not limited by electron donation from its redox partners.
FIG. 2.
Oxygen consumption by recombinant D. gigas ROO. (A) Representative oxygen consumption assay by D. gigas ROO monitored with a Clark-type electrode. FlRd reductase, E. coli flavorubredoxin reductase; Dg_Rd, D. gigas recombinant rubredoxin. (B) Hyperbolic dependence of the oxygen reductase activity of ROO on the concentration of rubredoxin. Inset, linear dependence of the oxygen reductase activity of ROO on the concentration of E. coli FlRd reductase (tested range, 100 to 800 nM) at a fixed rubredoxin concentration of 16 μM.
Nitric oxide reductase activity of recombinant D. gigas ROO.
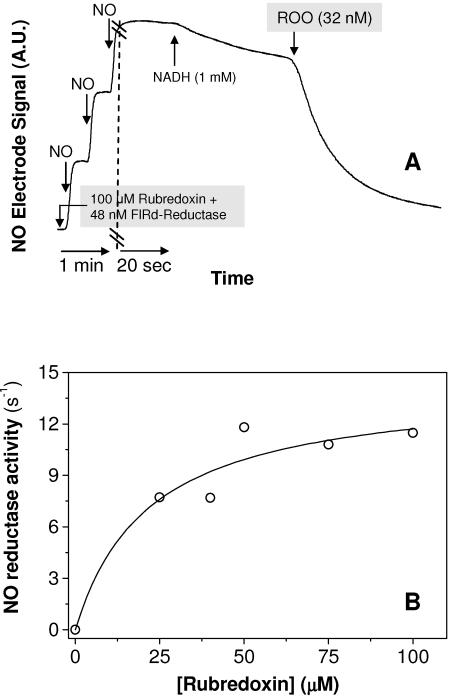
Nitric oxide consumption by recombinant ROO was measured anaerobically using excess reduced Rd as the electron donor to ROO. In the absence of oxygen, the expected NO reduction product was nitrous oxide (N2O), as corroborated by the NADH/NO stoichiometries observed for other FDPs (32) and our own unpublished results. In comparison with the O2 reduction measurements, two major differences reside in the fact that (i) NO reduction to nitrous oxide (N2O) required only two electrons (one per NO molecule) and (ii) the NO concentrations tested here were much lower (maximally 10 μM) than those of oxygen (∼240 μM). Thus, electron donation from reduced Rd should not pose the same experimental difficulties as for the O2 reduction assays, i.e., the slow rereduction of Rd by catalytic amounts of FlRd reductase. The NO reductase activity was thus studied as a function of reduced Rd concentration, by anaerobically incubating Rd with FlRd reductase and excess NADH prior to its addition to the reaction mixture. Upon addition of aliquots of a saturated NO solution (1.9 mM at 25°C), a slow NO consumption rate was observed, due to the unspecific reduction of NO by NADH, mediated by FlRd reductase (Fig. 3A). The NO reduction rates were shown to vary hyperbolically with increasing reduced Rd concentrations (Fig. 3B) with an estimated maximal NO consumption rate of 14.9 ± 3.2 mol NO · s−1 · mol ROO−1. From the observation of the NO consumption traces, an apparent Km can be estimated to be higher than that reported for E. coli FlRd and within the order of the values for the M. thermoacetica and D. vulgaris FDPs (32, 33), i.e., slightly >5 μM.
FIG. 3.
Nitric oxide consumption by recombinant D. gigas ROO. (A) Representative NO consumption anaerobic assay by D. gigas ROO monitored with an NO-specific electrode. (B) Hyperbolic dependence of NO reductase activity of ROO on the concentration of reduced rubredoxin.
Functional complementation.
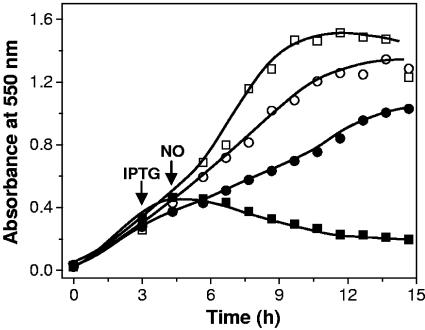
E. coli strain AG300, which contains the norV gene disrupted, is incapable of anaerobic growth in minimal medium containing low micromolar levels of added NO (13). To evaluate the NO reductase activity of ROO in vivo, growth of E. coli strain AG300 expressing plasmid-borne ROO was analyzed in the presence of NO. The results, depicted in Fig. 4, show that in contrast to the mutant strain, the strain expressing D. gigas ROO from the plasmid pFLAG-CTC(ROO) was capable of anaerobic growth under exposure to NO. This indicates that D. gigas ROO can replace E. coli FlRd, restoring anaerobic growth to the mutant strain under exposure to NO, most probably through its NO reductase activity.
FIG. 4.
Anaerobic growth of E. coli AG300 in the presence of 50 μM NO with expression of plasmid-borne D. gigas ROO. □, control strain AG300[pFLAG-CTC]; ▪, control strain AG300[pFLAG-CTC] with NO; ○, strain AG300[pFLAG-CTC(ROO)]; •, strain AG300[pFLAG-CTC(ROO)] with NO. IPTG and NO were added at the times following inoculation indicated by the arrows. Each data point is the average of three independent growth experiments.
Genotypic and phenotypic analysis of the D. gigas Δroo strain.
To clearly evaluate the ROO function in vivo, we constructed and characterized a D. gigas roo mutant. A plasmid containing the kanamycin resistance gene and the flanking regions of roo (Fig. 5) was constructed and used to transform wt D. gigas, as described in Materials and Methods. Following transformation and isolation of colonies grown in the presence of kanamycin, disruption of roo was confirmed by both PCR and Southern blot analyses.
PCR analysis revealed PCR products of the expected size in wt D. gigas and the mutant strain (data not shown). EcoRI-digested genomic DNAs from wild-type and Δroo D. gigas strains were hybridized with the roo and the kanamycin resistance gene probes. Hybridization with the roo probe resulted in two positive bands of the expected size (1,006 bp and 1,140 bp), only in the lane corresponding to wild-type D. gigas (data not shown). Hybridization with the kanamycin resistance gene probe resulted in a single positive band of the predicted size (2,351 bp) only in the lane corresponding to Δroo D. gigas (data not shown). The results obtained by both PCR and Southern blotting hence confirmed deletion of the roo gene and its replacement by the kanamycin resistance gene.
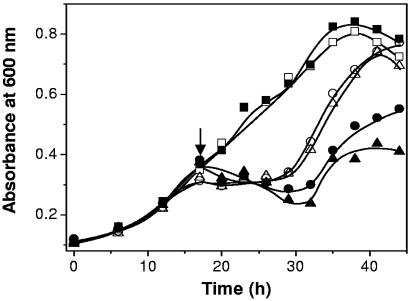
To determine the phenotype of the D. gigas Δroo strain, we first performed sensitivity assays of the presence of different concentrations of NO and the nitrosating agent GSNO with both the wild type and the mutant strain. A concentration of 10 μM NO or GSNO was shown to reveal growth differences between the two strains tested (Fig. 6). Under standard growth conditions, no differences were observed between wild-type and mutant strains. However, in the presence of either NO or GSNO, it is clear that growth of the mutant strain was severely impaired. These results strongly suggest that ROO is responsible for NO detoxification in D. gigas.
FIG. 6.
Growth curves of wild-type D. gigas and Δroo D. gigas in the presence of 10 μM NO or 10 μM GSNO. Open symbols, wt; solid symbols, Δroo; squares, no induced stress; circles, added NO; triangles, added GSNO. NO and GSNO were added at an initial concentration of 10 μM at the time following inoculation indicated by the arrow. Each data point is the average of three independent growth experiments.
The phenotype of the D. gigas Δroo strain in the presence of O2 was analyzed to address whether the activity of O2 reduction in vitro is significant in vivo. However, sensitivity assays performed in the presence of various concentrations of O2 in both wild-type and mutant strains, including full aeration of the cultures, revealed no growth differences between the two tested strains (data not shown).
roo expression analysis under stress conditions.
To correlate the previous results with roo expression, the roo mRNA levels in D. gigas were analyzed by real-time reverse transcription-PCR in the presence of NO, GSNO, and O2 (data not shown). roo was constitutively expressed, with a twofold induction of roo mRNA levels observed upon exposure to 1 mM GSNO but not upon direct exposure to NO. No significant changes in roo mRNA levels were detected upon exposure to 60 μM O2. The values obtained are the average of three independent experiments.
DISCUSSION
Prokaryotes such as Desulfovibrio spp. may face nitrosative stress when coexisting with denitrifiers, derived from the release of NO from denitrification reactions. Furthermore, several Desulfovibrio spp., possibly including D. gigas, inhabit the mammalian digestive tract, being related to chronic inflammatory diseases (14, 24). Within this environment, at the onset of infection and inflammatory processes they may face NO release from activated macrophages, as part of the immune system response of their hosts. Therefore, an anaerobe such as D. gigas must be able to cope with nitrosative stress, in addition to oxidative stress. Although D. gigas appears to have the appropriate enzymatic machinery to cope with oxidative stress derived from transient exposure to O2 and its reactive species (9, 10, 23, 34), no system for NO detoxification has been so far described for this organism. However, recent reports on D. gigas ROO orthologues endowed with NO reductase activity suggest a similar role for this enzyme.
In this work, we used multiple approaches to investigate whether D. gigas ROO is involved in NO reduction and/or oxygen reduction. The measurements of oxygen consumption by recombinant ROO in vitro showed that this FDP has a maximal oxygen reductase activity of 50.5 ± 10 mol O2 · s−1 · mol ROO−1 under the conditions tested. However, this activity can be underestimated, due to limitation by electron donation from its redox partners. Nevertheless, it is evident that D. gigas ROO can act as a robust oxygen reductase, pointing to a role for this protein as an efficient oxygen scavenger in this organism. With respect to NO, an apparent maximal consumption rate of 14.9 ± 3.2 mol NO · s−1 · mol ROO−1 was estimated. This rate was lower than those reported for other FDPs but still within those reported for NO reductases, including respiratory ones (40). These observations may imply that the electron transfer chain with ROO as its terminal reductase detoxifies either NO or O2 under different conditions.
Evidence of NO scavenging by D. gigas ROO in vivo came from the induced production of the protein in an E. coli strain containing a disrupted norV gene. Cultures grown anaerobically were no longer sensitive to NO or to the nitrosating agent GSNO. Most probably, the restoration of E. coli's ability to grow anaerobically in the presence of NO results from the lower NO levels, due to the reductase activity of the heterologously produced D. gigas ROO. Since in this experiment the rubredoxin-like module of FlRd (redox partner of ROO, Rd) was not provided and no Rd was encoded in the E. coli genome (4), an endogenous E. coli protein most likely acted as an electron provider for ROO. Similarly, in analogous experiments with M. thermoacetica and D. vulgaris in which the respective FDPs were expressed in the E. coli norV mutant strain in the absence of their redox partners, restoration of the anaerobic phenotype was also observed (32, 33).
To further complement these results on ROO function, we assessed the activity in vivo of ROO in D. gigas by phenotypic analysis of a mutant roo strain. This strain showed severe growth impairment upon exposure to 10 μM NO, compared to the wild type. Upon exposure to stress, both strains showed growth arrest, which was clearly resumed in the wild-type strain, while the mutant strain did not recover efficiently. The slight recovery of the mutant strain was probably due to removal of NO from the reducing medium.
This, as well as the aforementioned E. coli complementation results, supports the idea that ROO is indeed involved in NO detoxification as an NO reductase in D. gigas.
Under exposure to oxygen (up to 60 μM), no differences between the wild type and the mutant strain were observed. Given the reductive nature of the culture medium, it is possible that under these conditions the introduced 60 μM O2 was promptly reduced by the medium, with very little or no effect on the cultures. Furthermore, D. gigas was shown to have several enzymes besides ROO that allow it to deal with oxygen (9, 23, 34). The combination of these features contributes to the absence of phenotypic differences between wild-type and Δroo D. gigas strains in the presence of oxygen.
Analysis of roo gene expression in D. gigas revealed no significant changes in roo mRNA levels upon exposure to NO. On the contrary, when cells were exposed to the nitrosating agent GSNO, there was an increase in roo mRNA levels of approximately twofold. This seemingly contradictory result may be due to depletion of NO from the highly reductive growth medium to levels insufficient to cause induction of roo expression, whereas the GSNO concentration used seems to cause a nitrosative effect sufficient for induction of roo mRNA levels. Furthermore, some bacteria are much more sensitive to S-nitrosothiols (such as GSNO) than to NO (29), and this may also be the case with D. gigas.
Upon exposure to oxygen, no induction of roo mRNA levels was observed, although it cannot be excluded that the added oxygen may not have been enough to trigger a response, especially given the reductive nature of the growth medium.
These results suggest that under the conditions tested, NO but not O2 induces roo expression at the transcriptional level.
The mechanism by which expression of roo is regulated is not known; contrary to FlRd from E. coli, ROO is constitutively expressed in D. gigas (35), as its counterpart from D. vulgaris (33). No consensus sequences similar to those recognized by regulator proteins, such as FNR or ArcAB involved in the response to O2 or NO, were found in the rd-roo promoter region (35). In addition and contrary to what is observed with E. coli FlRd, no gene encoding a norR homologue, a putative physiological NO sensor in enteric bacteria (18, 25, 39), was found in the upstream vicinity of D. gigas ROO. However, two putative consensus sequences for a NorR-binding box [GT-(N7)-AC] were found upstream of the rd-roo coding region. According to genome database analyses, this inverted repeat is found in several bacteria upstream of gene loci encoding proteins involved in nitric oxide metabolism (6), which is in agreement with our results regarding the involvement of ROO in NO detoxification in D. gigas.
Altogether, our observations strongly suggest that D. gigas ROO may afford a protective mechanism for this organism when it faces either NO or oxygen stress.
Acknowledgments
E. coli strain AG300 was kindly provided by Paul R. Gardner (13).
This work has been supported by FCT (37480 to C.R.-P., 44597 and 37406 to M.T., SFRH/BD/5219/2001 to R.R., SFRH/BD/9136/2002 to J.B.V., and BIC 031/2003 to R.F.).
REFERENCES
- 1.Andersson, J. O., A. M. Sjogren, L. A. Davis, T. M. Embley, and A. J. Roger. 2003. Phylogenetic analyses of diplomonad genes reveal frequent lateral gene transfers affecting eukaryotes. Curr. Biol. 13:94-104. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. Greene Publishing Associates-Wiley Interscience, New York, N.Y.
- 3.Beckman, J. S., D. A. Wink, and J. P. Crow. 1996. Nitric oxide and peroxynitrite, p. 61-70. In M. Feelisch and J. S. Stamler (ed.), Methods in nitric oxide research. Wiley & Sons, Ltd., Chichester, United Kingdom.
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Broco, M., M. Rousset, S. Oliveira, and C. Rodrigues-Pousada. 2005. Deletion of flavoredoxin gene in Desulfovibrio gigas reveals its participation in thiosulfate reduction. FEBS Lett. 579:4803-4807. [DOI] [PubMed] [Google Scholar]
- 6.Busch, A., A. Pohlmann, B. Friedrich, and R. Cramm. 2004. A DNA region recognized by the nitric oxide-responsive transcriptional activator NorR is conserved in ß- and γ-proteobacteria. J. Bacteriol. 186:7980-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L., M. Y. Liu, J. Legall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas. Eur. J. Biochem. 216:443-448. [DOI] [PubMed] [Google Scholar]
- 8.Chen, L., M. Y. Liu, J. LeGall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 193:100-105. [DOI] [PubMed] [Google Scholar]
- 9.Dos Santos, W. G., I. Pacheco, M. Y. Liu, M. Teixeira, A. V. Xavier, and J. LeGall. 2000. Purification and characterization of an iron superoxide dismutase and a catalase from the sulfate-reducing bacterium Desulfovibrio gigas. J. Bacteriol. 182:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fareleira, P., B. S. Santos, C. Antonio, P. Moradas-Ferreira, J. LeGall, A. V. Xavier, and H. Santos. 2003. Response of a strict anaerobe to oxygen: survival strategies in Desulfovibrio gigas. Microbiology 149:1513-1522. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, D. S., and D. C. Price. 1964. A simple serum iron method using the new sensitive chromogen tripyridyl-S-triazine. Clin. Chem. 10:21-31. [PubMed] [Google Scholar]
- 12.Frazao, C., G. Silva, C. M. Gomes, P. Matias, R. Coelho, L. Sieker, S. Macedo, M. Y. Liu, S. Oliveira, M. Teixeira, A. V. Xavier, C. Rodrigues-Pousada, M. A. Carrondo, and J. Le Gall. 2000. Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat. Struct. Biol 7:1041-1045. [DOI] [PubMed] [Google Scholar]
- 13.Gardner, A. M., R. A. Helmick, and P. R. Gardner. 2002. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J. Biol. Chem. 277:8172-8177. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, G. R., J. H. Cummings, and G. T. McFarlane. 1991. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol. Ecol. 86:103-112. [Google Scholar]
- 15.Gomes, C. M., A. Giuffre, E. Forte, J. B. Vicente, L. M. Saraiva, M. Brunori, and M. Teixeira. 2002. A novel type of nitric-oxide reductase. Escherichia coli flavorubredoxin. J. Biol. Chem. 277:25273-25276. [DOI] [PubMed] [Google Scholar]
- 16.Gomes, C. M., G. Silva, S. Oliveira, J. LeGall, M. Y. Liu, A. V. Xavier, C. Rodrigues-Pousada, and M. Teixeira. 1997. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J. Biol. Chem. 272:22502-22508. [DOI] [PubMed] [Google Scholar]
- 17.Gomes, C. M., J. B. Vicente, A. Wasserfallen, and M. Teixeira. 2000. Spectroscopic studies and characterization of a novel electron-transfer chain from Escherichia coli involving a flavorubredoxin and its flavoprotein reductase partner. Biochemistry 39:16230-16237. [DOI] [PubMed] [Google Scholar]
- 18.Justino, M. C., J. B. Vicente, M. Teixeira, and L. M. Saraiva. 2005. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem. 280:2636-2643. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki, S., J. Ishikura, Y. Watamura, and Y. Niimura. 2004. Identification of O2-induced peptides in an obligatory anaerobe, Clostridium acetobutylicum. FEBS Lett. 571:21-25. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Lamosa, P., L. Brennan, H. Vis, D. L. Turner, and H. Santos. 2001. NMR structure of Desulfovibrio gigas rubredoxin: a model for studying protein stabilization by compatible solutes. Extremophiles 5:303-311. [DOI] [PubMed] [Google Scholar]
- 22.Legall, J., G. Mazza, and N. Dragoni. 1965. Cytochome C3 of Desulfovibrio gigas. Biochim. Biophys. Acta 99:385-387. [PubMed] [Google Scholar]
- 23.Lemos, R. S., C. M. Gomes, M. Santana, J. LeGall, A. V. Xavier, and M. Teixeira. 2001. The ‘strict’ anaerobe Desulfovibrio gigas contains a membrane-bound oxygen-reducing respiratory chain. FEBS Lett. 496:40-43. [DOI] [PubMed] [Google Scholar]
- 24.Loubinoux, J., F. M. Valente, I. A. Pereira, A. Costa, P. A. Grimont, and A. E. Le Faou. 2002. Reclassification of the only species of the genus Desulfomonas, Desulfomonas pigra, as Desulfovibrio piger comb. nov. Int. J. Syst. Evol. Microbiol. 52:1305-1308. [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhyay, P., M. Zheng, L. A. Bedzyk, R. A. LaRossa, and G. Storz. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. USA 101:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues, R., F. M. Valente, I. A. Pereira, S. Oliveira, and C. Rodrigues-Pousada. 2003. A novel membrane-bound Ech [NiFe] hydrogenase in Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 306:366-375. [DOI] [PubMed] [Google Scholar]
- 27.Rousset, M., L. Casalot, B. J. Rapp-Giles, Z. Dermoun, P. de Philip, J. P. Belaich, and J. D. Wall. 1998. New shuttle vectors for the introduction of cloned DNA in Desulfovibrio. Plasmid 39:114-122. [DOI] [PubMed] [Google Scholar]
- 28.Rousset, M., Z. Dermoun, M. Chippaux, and J. P. Belaich. 1991. Marker exchange mutagenesis of the hydN genes in Desulfovibrio fructosovorans. Mol. Microbiol. 5:1735-1740. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva, L. M., J. B. Vicente, and M. Teixeira. 2004. The role of the flavodiiron proteins in microbial nitric oxide detoxification. Adv. Microb. Physiol. 49:77-129. [DOI] [PubMed] [Google Scholar]
- 30.Sarti, P., P. L. Fiori, E. Forte, P. Rappelli, M. Teixeira, D. Mastronicola, G. Sanciu, A. Giuffre, and M. Brunori. 2004. Trichomonas vaginalis degrades nitric oxide and expresses a flavorubredoxin-like protein: a new pathogenic mechanism? Cell Mol. Life Sci. 61:618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sass, A. M., A. Eschemann, M. Kuhl, R. Thar, H. Sass, and H. Cypionka. 2002. Growth and chemosensory behavior of sulfate-reducing bacteria on oxygen-sulfide gradients. FEMS Microbiol. Ecol. 40:47-54. [DOI] [PubMed] [Google Scholar]
- 32.Silaghi-Dumitrescu, R., E. D. Coulter, A. Das, L. G. Ljungdahl, G. N. Jameson, B. H. Huynh, and D. M. Kurtz, Jr. 2003. A flavodiiron protein and high molecular weight rubredoxin from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry 42:2806-2815. [DOI] [PubMed] [Google Scholar]
- 33.Silaghi-Dumitrescu, R., K. Y. Ng, R. Viswanathan, and D. M. Kurtz, Jr. 2005. A flavo-diiron protein from Desulfovibrio vulgaris with oxidase and nitric oxide reductase activities. evidence for an in vivo nitric oxide scavenging function. Biochemistry 44:3572-3579. [DOI] [PubMed] [Google Scholar]
- 34.Silva, G., S. Oliveira, C. M. Gomes, I. Pacheco, M. Y. Liu, A. V. Xavier, M. Teixeira, J. Legall, and C. Rodrigues-Pousada. 1999. Desulfovibrio gigas neelaredoxin. A novel superoxide dismutase integrated in a putative oxygen sensory operon of an anaerobe. Eur. J. Biochem. 259:235-243. [DOI] [PubMed] [Google Scholar]
- 35.Silva, G., S. Oliveira, J. LeGall, A. V. Xavier, and C. Rodrigues-Pousada. 2001. Analysis of the Desulfovibrio gigas transcriptional unit containing rubredoxin (rd) and rubredoxin-oxygen oxidoreductase (roo) genes and upstream ORFs. Biochem. Biophys. Res. Commun. 280:491-502. [DOI] [PubMed] [Google Scholar]
- 36.Smith, P. K., G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 37.Stamler, J. S., D. I. Simon, J. A. Osborne, M. E. Mullins, O. Jaraki, T. Michel, D. J. Singel, and J. Loscalzo. 1992. S-Nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 89:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susin, S., J. Abian, F. Sanchez-Baeza, M. L. Peleato, A. Abadia, E. Gelpi, and J. Abadia. 1993. Riboflavin 3′- and 5′-sulfate, two novel flavins accumulating in the roots of iron-deficient sugar beet (Beta vulgaris). J. Biol. Chem. 268:20958-20965. [PubMed] [Google Scholar]
- 39.Tucker, N. P., B. D'Autreaux, D. J. Studholme, S. Spiro, and R. Dixon. 2004. DNA binding activity of the Escherichia coli nitric oxide sensor NorR suggests a conserved target sequence in diverse proteobacteria. J. Bacteriol. 186:6656-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]