Abstract
Expression of mucD, encoding a homologue of the HtrA(DegP) family of endoserine proteases, was investigated in Pseudomonas aeruginosa. Expressed from the algT-mucABCD operon, MucD was detected in mucoid (FRD1) and nonmucoid (PAO1) parental strains and also when polar insertions were placed upstream in algT or mucB. A transcriptional start site for a mucD promoter (PmucD) was mapped within mucC. Expression of single-copy mucD217, encoding MucD altered in the protease motif (S217A), was defective in temperature resistance and alginate gene regulation.
Pseudomonas aeruginosa strains causing chronic pulmonary tract infections in cystic fibrosis (CF) patients typically show a highly mucoid phenotype, indicating production of the exopolysaccharide alginate (14). The activation of alginate genes is complex (11, 26). Briefly, the algT gene (algU), which is essential for alginate production (8, 9), encodes the alternative sigma factor σ22, which has homology to the extracytoplasmic function sigma E (σE) of Escherichia coli (6, 7, 15, 22). The algT operon consists of algT-mucA-mucB-mucC-mucD. MucA is an anti-sigma factor that sequesters σ22 (19, 27), and mutations in mucA are common in mucoid P. aeruginosa isolates from CF patients (17). MucB (AlgN) is a periplasmic, negative regulator (10, 16, 19). The role of MucC is unclear. MucD is a close homologue of the E. coli periplasmic serine protease HtrA (DegP) (3, 24), which is required in E. coli for resistance to high temperature and oxidative stress (25). HtrA degrades misfolded periplasmic proteins at elevated temperatures (12) and has a chaperone function at lower temperatures (33). Most organisms have genes for one or more HtrA-like proteases.
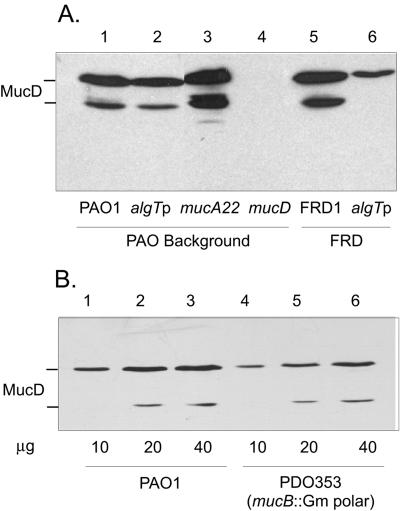
The rpoE and algT operons, where σE is under the control of anti-sigma RseA and periplasmic RseB, are similar (5, 21). The obvious difference in these operons is the location of the htrA/mucD gene. In P. aeruginosa, mucD is in the algT operon, and in E. coli, htrA is unlinked but is under σE transcriptional control. This interesting difference led us to examine mucD regulation and function in P. aeruginosa. To investigate this, rabbit polyclonal antibodies (Covance Research Products) were raised to purified His-tagged MucD (Novagen) in order to detect MucD protein by chemiluminescence in immunoblots of proteins from cell lysates separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two strains were examined: wild-type (nonmucoid) PAO1 and a mucA22 (mucoid) strain, FRD1, from a CF patient (23). The detection of MucD was not dependent upon alginate production in that both mucoid and nonmucoid strains contained MucD as a prominent band of ∼48 kDa, with another less-intense band of lower molecular mass (Fig. 1A, lanes 1 and 5). In E. coli, mature HtrA (48 kDa) undergoes partial autocleavage under reducing conditions at the N terminus with the formation of two ∼43-kDa truncated polypeptides (12, 31, 32). Mature MucD (48 kDa) in P. aeruginosa, with 57% similarity to HtrA, presumably undergoes a similar processing phenomenon. MucD was not observed in the mucD mutant, PDO350 (lane 4), showing that the antibodies were specific for MucD. Compared to PAO1 (lane 1), its mucoid mucA22 derivative PDO300 (lane 3) contained more MucD, probably due to increased algT promoter activity (19). Interestingly, a polar insertion in algT in PAO1 (lane 2) or in FRD1 (lane 6) did not block production of MucD, although levels were reduced compared to parental strains. Since these polar insertions block transcription of mucD from the algT promoter (PalgT), another promoter (PmucD) apparently allowed for mucD expression, which was not σ22 (i.e., algT) dependent.
FIG. 1.
Western blot analyses of MucD in P. aeruginosa. (A) Effect of algT polar insertions (algTp) on detection of MucD in strains of PAO and FRD backgrounds. Cultures were grown in L broth to an optical density of 600 nm (OD600) of 1.0 and lysed. Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were probed with rabbit MucD antibodies. Lanes: 1, PAO1 (wild type); 2, PDO-LS586 (algT::aacCIΩ) (29); 3, PDO300 (mucA22; mucoid) (18); 4, PDO350 (ΔmucD::aacCIΩ, mucoid); 5, FRD1 (wild type, mucA22, mucoid) (23); 6, FRD440 (algT::Tn501) (9). MucD protein was detected in all strains except PDO350 (ΔmucD). (B) Effect of a polar insertion in mucB on downstream mucD expression and MucD protein levels. Culturing and immunodetection methods were the same as described for panel A. Samples of PAO1 (10, 20, or 40 μg protein in lanes 1, 2, and 3, respectively) or PDO353 mucB::aacCIΩ (10, 20, or 40 μg protein in lanes 4, 5, and 6, respectively) were compared. Densitometry (performed in triplicate) was used to determine cellular levels of MucD protein.
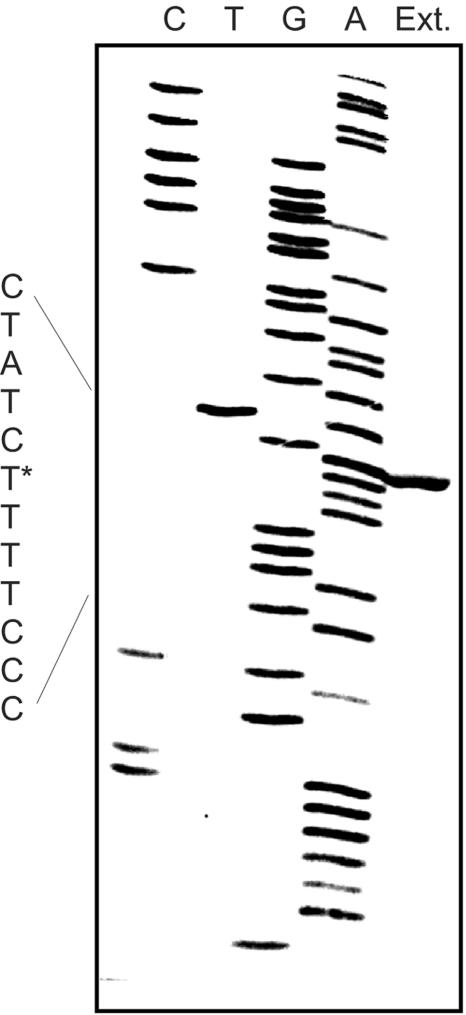
In E. coli, disruption of rpoE results in compensatory mutation(s) (4). To avoid this possibility with algT mutations in P. aeruginosa, a PAO1 mucBΩ polar mutant (PDO353) was constructed using an aacCIΩ polar (gentamicin resistance) cassette (28) and allelic replacement to again examine the expression of mucD downstream. Semiquantitative Western analysis (Fig. 1B) showed that this polar insertion also permitted production of MucD at ∼43% of the level in PAO1 as determined by densitometry. Thus, PmucD expression leads to about half of the amount of MucD in the cell during exponential growth. When primer extension analysis (1) was used, a primer complementary to DNA 165 bases upstream of the mucD start codon produced a product that began at nucleotide −245 relative to the start codon of the mucD open reading frame (Fig. 2). This placed the start of PmucD transcription in the middle of mucC.
FIG. 2.
Results of a primer extension (Ext.) analysis to determine the transcriptional initiation site of mucD transcripts from PmucD. Total cellular RNA was isolated (QIAGEN RNeasy) from PAO1 cultures (L broth; OD600 of 1.0). A primer (5′ACGATGATCAGAGGTTCGACAAGGCCCG) complementary to the region of DNA 165 bases upstream of the mucD ATG start codon was end labeled with [γ-32P]ATP (Perkin-Elmer), annealed to 25 μg of RNA, and extended with AMV reverse transcriptase (New England Biolabs). The resulting extension products were loaded onto an 8% polyacrylamide-7 M urea gel. An adjacent sequencing ladder (CTGA) was prepared using a USB Thermo Sequenase 33P-radiolabeled terminator cycle sequencing kit (USB Corp.) and the same oligonucleotide. Products were revealed by autoradiography. The 5′ end of the extension product for PmucD aligned with a T*, located at −245 bp and within mucC.
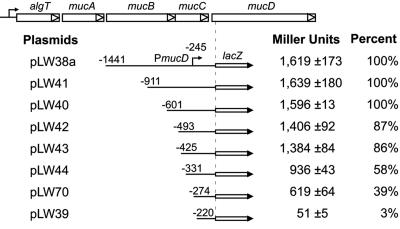
To evaluate the PmucD promoter region, mucD-lacZ reporter fusions were constructed in lacZ vector pSS223 (34) and tested for β-galactosidase expression (measured in Miller units) in PAO1. Each plasmid had the same 3′ fusion joint, 62 nucleotides into the mucD coding region, but with various lengths of DNA upstream (Fig. 3). The largest construct (pLW38a) contained mucB-mucC-mucD′-lacZ and showed high activity (∼1,600 U), even though it did not contain the upstream PalgT. With this fragment in reverse orientation to lacZ, β-galactosidase activity was less than 1% of that seen with pLW38a, indicating that the background was low. The constructs with just mucB deletions (pLW41, pLW40, pLW42, and pLW43) showed little or no change in maximum mucD-lacZ activity. Further deletions in the mucC gene (pLW44 and pLW70) showed some deleterious effects. Interestingly, having only 29 bp upstream of the PmucD transcriptional start site (pLW70) still allowed 39% of maximal PmucD-lacZ activity, but a deletion of the start site (pLW39) reduced β-galactosidase activity to 3% of the maximum. Thus, the DNA requirement for PmucD expression was small. When PAO-derived mutants with defects in known regulators for alginate production (e.g., algT, mucA, mucB, mucD, algB, kinB, and rpoS) were used as hosts for pLW43 (i.e., PmucD-lacZ), there was no observable effect on β-galactosidase activity, indicating that PmucD was not under their control (in L broth at log phase; data not shown). Since mucD mutants are temperature sensitive (2), the effect of heat stress on PmucD was also investigated. The growth temperature of PAO1(pLW43) was shifted from 37°C to 44°C, but again there was no measurable effect on the expression β-galactosidase activity for PmucD-lacZ, suggesting that PmucD was not heat shock inducible (data not shown).
FIG. 3.
Deletion analysis of DNA for relative PmucD expression. A series of lacZ transcriptional fusions were constructed using the lacZ transcriptional fusion vector pSS223 (34) with the 3′ end at +60 bp into the mucD coding sequence and with various amounts of upstream DNA. Plasmids were transferred to PAO1 by conjugation and grown in L broth to an OD600 of 1.0, and aliquots were tested for β-galactosidase activity (Miller units). Activity from pLW38a containing the mucB-mucC region upstream of mucD was assigned a value of 100%.
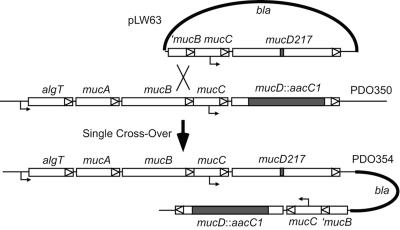
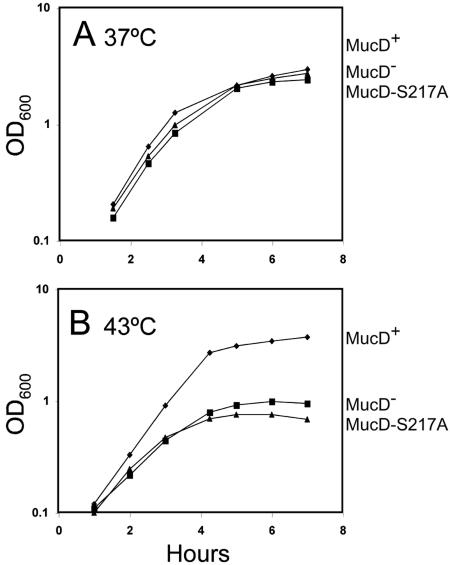
On the basis of the homology MucD shares with HtrA(DegP) and the temperature-sensitive phenotype in both mutant backgrounds, MucD is likely to be a protease involved in the proteolysis of misfolded periplasmic proteins, although this activity has not yet been directly demonstrated. We took advantage of the well-defined His, Asp, and Ser catalytic triad known for HtrA-like serine proteases (30) to construct the mucD217 allele by oligonucleotide mutagenesis, which encoded MucD-S217A, substituted in the enzymatic motif. This was cloned into a suicide vector (pUC19) and crossed into the PDO350 chromosome so that the resulting strain (PDO354) would express mucD217 in single copy numbers in the algT operon (Fig. 4), and MucD-S217A was observed using Western blot analysis (data not shown). When strains were compared for growth in L broth at 37°C, the MucD+, MucD−, and MucD-S217A strains grew equally well (Fig. 5A). In contrast, at 43°C the MucD− and MucD-S217A strains showed markedly reduced growth compared to PAO1 (Fig. 5B). Similar results were observed at 37°C and 43°C when the mucD defect in PDO350 was complemented from a multicopy plasmid with wild-type mucD (pLW37) or with mucD217 (pLW67) (data not shown). These results were similar to those seen in E. coli (30), suggesting that MucD proteolytic activity was also required for normal growth at elevated temperatures.
FIG. 4.
Construction of PDO354 with mucD217 expressing MucD-S217A with a defect in the conserved protease motif in single copy from the chromosome. Oligonucleotide mutagenesis as described previously (20) was used with pLW1 (pUC19 with “mucB mucC mucD lep” [2.5 kb]) to form pLW63 with the mucD217 allele. Suicide plasmid pLW63 was transferred to PDO350 (ΔmucD::aacCIΩ, mucoid), with selection for carbenicillin (bla) resistance to mediate integration into the chromosome by homologous recombination. The resulting strain, PDO354, was verified by PCR for correct insertion in the chromosomal operon, and production of MucD-S217A was confirmed by Western analysis.
FIG. 5.
Comparison of the growth of PAO isogenic strains at 37°C (A) or 43°C (B) expressing single-copy mucD+, ΔmucD, or mucD217 alleles. Growth was monitored by OD600, and data shown are representative of three comparable experiments. Symbols indicate the following strains: ⧫, PAO1 mucD+; ▪, PDO350 ΔmucD; and ▴, PDO354 mucD217.
A mucD null mutant also produces alginate when grown on Pseudomonas isolation agar (2). To determine whether MucD's proteolytic activity was involved in the control of alginate production, we measured the amount of alginate (13) produced by the MucD+, MucD−, and MucD-S217A strains at 37°C. A mucA::aacCI (nonpolar) mucoid mutant (PDO351) was also included for comparison. PAO1 produced no detectable alginate, but the mucA, mucD, and mucD217 mutant strains all produced high levels of alginate (Table 1). Thus, MucD's proteolytic motif was important for its regulation of alginate production as well as temperature resistance. However, when mucD217 was expressed from a high-copy-number plasmid in the mucD null mutant, the control of alginate gene expression was restored (Table 1), suggesting that MucD has a second function only observed at higher levels. This may be similar to HtrA in E. coli, which is known to have both proteolytic and chaperone functions (33).
TABLE 1.
Alginate production by mucD mutantsa
| Strain | Genotype | Alginate |
|---|---|---|
| PAO1 | mucD+ | BD |
| PDO351 | mucA::aacCI (nonpolar) | 41.75 ± 12.8 |
| PDO350 | mucD::aacCIΩ | 58.42 ± 6.79 |
| PDO354 | mucD217 | 44.82 ± 4.64 |
| PDO350(pLW37) | mucD+ (multicopy) | BD |
| PDO350(pLW67) | mucD217 (multicopy) | BD |
Derivatives of P. aeruginosa PAO1 (wild type) were grown on PIA plates for 48 h at 37°C, and then bacteria and alginate were harvested. The aacCI (gentamicin resistance) cassettes were from Schweizer (28). Plasmids pLW37 and pLW67 were vector pASK-IBA3 (Sigma) containing oriV(SF) for multi-copy, broad-host-range replication (34) and either mucD+ and mucD217, respectively. Alginate was measured in milligrams per milligram of wet cell weight by use of a colorimetric assay (13). BD means that alginate levels were below detection, indicating that the mucD allele was still functioning as a negative regulator.
Acknowledgments
We are grateful to Sang-jin Suh and Laura Silo-Suh (of our laboratory) for providing some of the mutants.
This work was supported by Public Health Service grant AI-19146 from the National Institute of Allergy and Infectious Disease (D.E.O.) and in part by Veterans Administration medical research funds (D.E.O).
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1993. Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., Hoboken, N.J.
- 2.Boucher, J. C., J. Martinez-Salazar, M. J. Schurr, M. H. Mudd, H. Yu, and V. Deretic. 1996. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J. Bacteriol. 178:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, J. C., H. Yu, M. H. Mudd, and V. Deretic. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect. Immun. 65:3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Las Peñas, A., L. Connolly, and C. A. Gross. 1997. σE is an essential sigma factor in Escherichia coli. J. Bacteriol. 179:6862-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Las Penas, A., L. Connolly, and C. A. Gross. 1997. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigma E. Mol. Microbiol. 24:373-385. [DOI] [PubMed] [Google Scholar]
- 6.Deretic, V., M. J. Schurr, J. C. Boucher, V. Deretic, M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVries, C. A., and D. E. Ohman. 1994. Mucoid to nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternative sigma factor, and shows evidence for autoregulation. J. Bacteriol. 176:6677-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J. L., and D. E. Ohman. 1988. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J. Bacteriol. 170:1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., and D. E. Ohman. 1988. Use of a gene replacement cosmid vector for cloning alginate conversion genes from mucoid and nonmucoid Pseudomonas aeruginosa strains: algS controls expression of algT. J. Bacteriol. 170:3228-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg, J. B., W. L. Gorman, J. L. Flynn, and D. E. Ohman. 1993. A mutation in algN permits trans-activation of alginate production by algT in Pseudomonas species. J. Bacteriol. 175:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain, S., and D. E. Ohman. 2004. Alginate biosynthesis., p. 53-81. In J.-L. Ramos (ed.), Pseudomonas, vol. 3 (biosynthesis of macromolecules and molecular metabolism). Kluwer Academic/Plenum Publishers, New York, N.Y. [Google Scholar]
- 12.Kim, K. I., S. C. Park, S. H. Kang, G. W. Cheong, and C. H. Chung. 1999. Selective degradation of unfolded proteins by the self-compartmentalizing HtrA protease, a periplasmic heat shock protein in Escherichia coli. J. Mol. Biol. 294:1363-1374. [DOI] [PubMed] [Google Scholar]
- 13.Knutson, C. A., and A. Jeanes. 1968. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal. Biochem. 24:470-481. [DOI] [PubMed] [Google Scholar]
- 14.Linker, A., and R. S. Jones. 1966. A new polysaccharide resembling alginic acid isolated from pseudomonads. J. Biol. Chem. 241:3845-3851. [PubMed] [Google Scholar]
- 15.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of Streptomyces coelicolor SigE gene reveals the existence of a subfamily of eubacterial RNA polymerase σ factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, D. W., M. J. Schurr, M. H. Mudd, and V. Deretic. 1993. Differentiation of Pseudomonas aeruginosa into the alginate-producing form: inactivation of mucB causes conversion to mucoidy. Mol. Microbiol. 9:497-506. [DOI] [PubMed] [Google Scholar]
- 17.Martin, D. W., M. J. Schurr, M. H. Mudd, J. R. W. Govan, B. W. Holloway, and V. Deretic. 1993. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 90:8377-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathee, K., O. Ciofu, C. K. Sternberg, P. Lindum, J. Campbell, P. Jensen, A. Johnsen, M. Givskov, D. Ohman, S. Molin, N. Høiby, and A. Kharazmi. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145:1349-1357. [DOI] [PubMed] [Google Scholar]
- 19.Mathee, K., C. J. McPherson, and D. E. Ohman. 1997. Posttranslational control of the algT (algU)-encoded σ22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). J. Bacteriol. 179:3711-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael, S. F. 1994. Mutagenesis by incorporation of a phosphorylated oligo during PCR amplification. BioTechniques 16:410-412. [PubMed] [Google Scholar]
- 21.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli sigmaE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355-371. [DOI] [PubMed] [Google Scholar]
- 22.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 23.Ohman, D. E., and A. M. Chakrabarty. 1981. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 33:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohman, D. E., K. Mathee, C. J. McPherson, C. A. DeVries, S. Ma, D. J. Wozniak, and M. J. Franklin. 1996. Regulation of the alginate (algD) operon in Pseudomonas aeruginosa, p. 472-483. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. American Society for Microbiology, Washington, D.C.
- 25.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey, D. M., and D. J. Wozniak. 2005. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 56:309-322. [DOI] [PubMed] [Google Scholar]
- 27.Schurr, M. J., H. Yu, J. M. Martinez-Salazar, J. C. Boucher, and V. Deretic. 1996. Control of AlgU, a member of the σE-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J. Bacteriol. 178:4997-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweizer, H. P. 1993. Small broad-host-range gentamicin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-833. [PubMed] [Google Scholar]
- 29.Silo-Suh, L., S. J. Suh, P. A. Sokol, and D. E. Ohman. 2002. A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proc. Natl. Acad. Sci. USA 99:15699-15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorko-Glonek, J., A. Wawrzynow, K. Krzewski, K. Kurpierz, and B. Lipinska. 1995. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene 163:47-52. [DOI] [PubMed] [Google Scholar]
- 31.Skorko-Glonek, J., D. Zurawa, E. Kuczwara, M. Wozniak, Z. Wypych, and B. Lipinska. 1999. The Escherichia coli heat shock protease HtrA participates in defense against oxidative stress. Mol. Gen. Genet. 262:342-350. [DOI] [PubMed] [Google Scholar]
- 32.Skorko-Glonek, J., D. Zurawa, F. Tanfani, A. Scire, A. Wawrzynow, J. Narkiewicz, E. Bertoli, and B. Lipinska. 2003. The N-terminal region of HtrA heat shock protease from Escherichia coli is essential for stabilization of HtrA primary structure and maintaining of its oligomeric structure. Biochim. Biophys. Acta 1649:171-182. [DOI] [PubMed] [Google Scholar]
- 33.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 34.Suh, S. J., L. A. Silo-Suh, and D. E. Ohman. 2004. Development of tools for the genetic manipulation of Pseudomonas aeruginosa. J. Microbiol. Methods 58:203-212. [DOI] [PubMed] [Google Scholar]